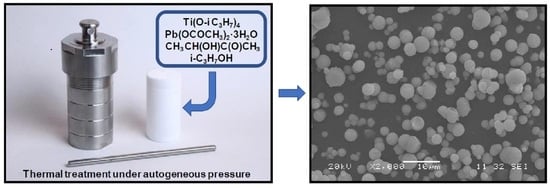
Novel Sol-Gel Synthesis of Spherical Lead Titanate Submicrometer Powders
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Morphology of PbTiO3 Powders
3.2. Crystalline Phase Development
3.3. Proposed Particle Growth Mechanism
- The use of acetoin as a ligand and the autoclave as the reaction medium are essential to form PT spherical particles.
- Nanometric primary PT particulates directly formed under autogenous pressure.
- The nanosized primary particulates weakly bond and confine within submicrometer spherical powders.
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atuchin, V.V.; Galashov, E.N.; Kozhukhov, A.S.; Pokrovsky, L.D.; Shlegel, V.N. Epitaxial growth of ZnO nanocrystals at ZnWO4 (0 1 0) cleaved surface. J. Cryst. Growth 2011, 318, 1147–1150. [Google Scholar] [CrossRef]
- Mudavakkat, V.H.; Atuchin, V.V.; Kruchinin, V.N.; Kayani, A.; Ramana, C.V. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. [Google Scholar] [CrossRef]
- Sylenko, P.M.; Shlapak, A.M.; Petrovska, S.S.; Khyzhun, O.Y.; Solonin, Y.M.; Atuchin, V.V. Direct nitridation synthesis and characterization of Si3N4 nanofibers. Res. Chem. Intermed 2015, 41, 10037–10048. [Google Scholar] [CrossRef]
- Bilir, G.; Erguzel, O. Up-conversion emission properties and unexpected white light emission from Er3+/Yb3+ doped Gd2O3 nanophosphors. Mater. Res. Express 2016, 3, 106201. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Lvanova, S.; Vasilchenco, N.; Atuchin, V.; Korolkov, I.; Russakov, D.; Prosekov, A. Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int. Biodeter. Biodeg. 2020, 146, 104821. [Google Scholar] [CrossRef]
- Suzuki, M.; Uedaira, S.; Masuya, H.; Tamura, H. Ceramics Transactions, Vol. 1, Ceramic Powder Science II; Messing, G.L., Fuller, E.R., Jr., Hausner, H., Eds.; The American Ceramic Society Inc.: Westerville, OH, USA, 1988; p. 163. [Google Scholar]
- Cheng, H.; Ma, J.; Zhao, Z.; Qiang, D.; Li, Y.; Yao, X. Hydrothermal Synthesis of Acicular Lead Titanate Fine Powders. J. Am. Ceram. Soc. 1992, 75, 1123. [Google Scholar] [CrossRef]
- Beal, K.C. Advances in Ceramics, Vol. 21, Ceramic Powder Science; Messing, G.L., Mazdiyasni, K.S., McCauley, J.W., Haber, R.A., Eds.; The American Ceramic Society Inc.: Westerville, OH, USA, 1987; p. 33. [Google Scholar]
- Moon, J.; Li, T.; Adair, J.H. Low Temperature Synthesis of Lead Titanate by a Hydrothermal Method. J. Mater. Res. 1997, 12, 189. [Google Scholar] [CrossRef]
- Choa, S.-B.; Noha, J.-S.; Lenckab, M.M.; Rimanc, R.E. Low Temperature Hydrothermal Synthesis and Formation Mechanisms of Lead Titanate (PbTiO3) Particles Using Tetramethylammonium Hydroxide: Thermodynamic Modelling and Experimental Verification. J. Eur. Ceram. Soc. 2003, 23, 2323–2335. [Google Scholar] [CrossRef]
- Ohara, Y.; Koumoto, K.; Shimizu, T.; Yanagida, H. Hydrotherrnal Synthesis of Fibrous Lead Titanate Powders. J. Mater. Sci. 1995, 30, 263–266. [Google Scholar] [CrossRef]
- Hu, Y.M.; Gu, H.S.; Sun, X.C.; You, J.; Wang, J. Photoluminescence and Raman Scattering Studies on PbTiO3 Nanowires Fabricated by Hydrothermal Method at Low Temperature. Appl. Phys. Lett. 2006, 88, 193120. [Google Scholar] [CrossRef]
- Ye, Z.; Slamovich, E.B.; King, A.H. Size-Driven Domain Reorientation in Hydrothermally Derived Lead Titanate Nanoparticles. J. Mater. Res. 2005, 20, 558. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E. Morphological control of particles. Curr. Opin. Colloid Interface Sci. 2000, 5, 160–167. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, C.H.; Kim, D.K. Hydrothermal Synthesis of Spherical Perovskite Oxide Powders Using Spherical Gel Powders. J. Am. Ceram. Soc. 1998, 81, 1353–1356. [Google Scholar] [CrossRef]
- Rhodes, W.H. Agglomerate and Particle Size Effects on Sintering Ytttra-Stabilized Zirconia. J. Am. Ceram. Soc. 1981, 64, 19–22. [Google Scholar] [CrossRef]
- Funakubo, H.; Shinozaki, K.; Mizutani, N. Formability and Sinterability of Hydrothermally Crystallized Monodispersed Titanium Dioxide Particles. J. Ceram. Soc. Jpn. 1995, 103, 552–556. [Google Scholar]
- Cai, Z.; Xing, X.; Yu, R.; Sun, X.; Guirong, G. Morphology-Controlled Synthesis of Lead Titanate Powders. Inorg. Chem. 2007, 46, 7423–7427. [Google Scholar] [CrossRef]
- Chien, A.T.; Sachleben, J.; Kim, J.H.; Speck, J.S.; Lange, F.F. Synthesis and characterization of PbTiO3 powders and heteroepitaxial thin films by hydrothermal synthesis. J. Mater. Res. 1999, 14, 3303. [Google Scholar] [CrossRef]
- Moon, J.; Kerchner, J.A.; Krarup, H.; Adair, J.H. Hydrothermal synthesis of ferroelectric perovskites from chemically modified titanium isopropoxide and acetate salts. J. Mater. Res. 1999, 14, 425–435. [Google Scholar] [CrossRef]
- Moon, J.; Carasso, M.L.; Krarup, H.G.; Kerchner, J.A.; Adaira, J.H. Particle-shape control and formation mechanisms of hydrothermally derived lead titanate. J. Mater. Res. 1999, 14, 866. [Google Scholar] [CrossRef]
- Peterson, C.R.; Slamovich, E.B. Effect of processing parameters on the morphology of Hydrothermally derived PbTiO3 powders. J. Am. Ceram. Soc. 1999, 82, 1702. [Google Scholar] [CrossRef]
- Komarneni, S.; Li, Q.; Stefansson, K.M.; Roy, R. Microwave-hydrothermal processing for synthesis of electroceramic powders. J. Mater. Res. 1993, 8, 3176. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Jamieson, A.M.; Sirivat, A.; Wongkasemjit, S. A Novel Route to Perovskite Lead Titanate From Lead and Titanium Glycolates Via the Sol–Gel Process. Appl. Organometal. Chem. 2006, 20, 886–894. [Google Scholar] [CrossRef]
- Tangboriboona, N.; Jamiesonb, A.; Sirivata, A.; Wongkasemjita, S. A Novel Route to Perovskite Lead Zirconate Titanate From Glycolate Precursors Via the Sol—Gel Process. Appl. Organometal. Chem. 2008, 22, 104–113. [Google Scholar] [CrossRef]
- Reaney, I.M.; Brooks, K.; Klissurska, R.; Pawlaczyk, C.; Setter, N. Use of Transmission Electron Microscopy for the Characterization of Rapid Thermally Annealed, Solution-Gel, Lead Zirconate Titanate Films. J. Am. Ceram. Soc. 1994, 77, 1209. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R.; Tahar, R.B.; Salah, A.B. Low-Temperature Processing and Characterization of Single-Phase PZT Powders by Sol-Gel Method. J. Mater. Sci. 2007, 42, 9801–9806. [Google Scholar] [CrossRef]
- Takagi, T.; Chiang, Y.-M. Origin of grain boundary weak links in BaPb1−xBixO3 superconductor. J. Appl. Phys. 1990, 68, 5750. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R. Structural and Electro-optical Properties of Sol-Gel Processed Cd2SnO4 Powder and Nanocrystalline Films. Thin Solid Film. 2017, 626, 85–93. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R.; Bel-Hadj-Tahar, N.; Salah, A.B. Preparation and characterization of PZT solid solutions via sol-gel process. J. Cryst. Growth 2007, 307, 40–43. [Google Scholar] [CrossRef]
- Li, S.; Condrate, R.A.; Spriggs, R.M. The FTIR and Raman spectra of lead zirconate titanate (PZT) prepared by sol-gel process. J. Can. Ceram. Soc. 1988, 57, 61–65. [Google Scholar]
- Budd, K.D.; Dey, S.K.; Payne, D.A. Sol gel processing of PbTiO3, PbZrO3, PZT, and PLZT thin films. Br. Ceram. Proc. 1985, 36, 107–121. [Google Scholar]
- Bel-Hadj-Tahar, R.; Abboud, M. Structural Development and Kinetic Analysis of PbTiO3 Powders Processed at Low-Temperature Via New Sol-Gel Approach. Sol. Stat. Sci. 2018, 78, 74–85. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R.; Abboud, M.; Bouzitoun, M.; Bouzitoun, M. Thermal Analysis of The Crystallization Kinetics of Lead Zirconate Titanate Powders Prepared Via Sol—Gel Route. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Klug, M.P.; Alexander, L.E. X-Ray Diffraction Procedure for Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1974; p. 634. [Google Scholar]
- Han, K.R.; Koo, H.J.; Hong, M.-J.; Lim, C.S. Simple Synthesis of Submicrometer Lead Titanate Powder by Precipitation of TiO2 Precursor on PbO Particulates. J. Am. Ceram. Soc. 2000, 83, 971–973. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R.; BelHadj-Tahar, N.; Mohamed, A.B. Sol-gel processing of highly transparent conducting Cd2SnO4 thin films. Eur. Phys. J. Appl. Phys. 2015, 69, 30302. [Google Scholar] [CrossRef]
- Bel-Hadj-Tahar, R.; Abboud, M.; Tahar, N.B. Microstructural and electrical properties of nanostructured leadzirconate titanate composite thick films processed for MEMS applications via hybrid sol-gel approach. J. Alloys Compd. 2020, 830, 154695. [Google Scholar] [CrossRef]
- Johnson, P.R.; White, J.D. Condensation of crotonic and tiglic acid dianions with aldehydes and ketones. J. Org. Chem. 1984, 49, 4424–4429. [Google Scholar] [CrossRef]
- Kessler, V.G.; Spijksma, G.I.; Seisenbaeva, G.A.; Hakansson, S.; Blank, D.H.A.; Bouwmeester, H.J.M. New insight in the role of modifying ligands in the sol-gel processing of metal alkoxide precursors: A possibility to approach new classes of materials. J. Sol.-Gel. Sci. Technol. 2006, 40, 163–179. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Kessler, V.G. Precursor directed synthesis—“Molecular” mechanisms in the Soft Chemistry approaches and their use for template-free synthesis of metal, metal oxide and metal chalcogenide nanoparticles and nanostructures. Nanoscale 2014, 6, 6229–6244. [Google Scholar] [CrossRef]











| Metal Concentration (mol/L) | Molar Ratio | Appearance | Stability |
|---|---|---|---|
| 0.4 | Acetnin:Ti = 2 Pb:Ti = 1 | Yellow | About 6 momths |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel-Hadj-Tahar, R.; Abboud, M.; Shkir, M.; Alhanash, A.; M. Anqi, A.E.; Belhadj Tahar, N.; Bouzitoun, M.; Benaissa, M. Novel Sol-Gel Synthesis of Spherical Lead Titanate Submicrometer Powders. Crystals 2021, 11, 484. https://doi.org/10.3390/cryst11050484
Bel-Hadj-Tahar R, Abboud M, Shkir M, Alhanash A, M. Anqi AE, Belhadj Tahar N, Bouzitoun M, Benaissa M. Novel Sol-Gel Synthesis of Spherical Lead Titanate Submicrometer Powders. Crystals. 2021; 11(5):484. https://doi.org/10.3390/cryst11050484
Chicago/Turabian StyleBel-Hadj-Tahar, Radhouane, Mohamed Abboud, Mohd. Shkir, Abdullah Alhanash, Ali Eisa M. Anqi, Noureddine Belhadj Tahar, Mouna Bouzitoun, and Mhamed Benaissa. 2021. "Novel Sol-Gel Synthesis of Spherical Lead Titanate Submicrometer Powders" Crystals 11, no. 5: 484. https://doi.org/10.3390/cryst11050484
APA StyleBel-Hadj-Tahar, R., Abboud, M., Shkir, M., Alhanash, A., M. Anqi, A. E., Belhadj Tahar, N., Bouzitoun, M., & Benaissa, M. (2021). Novel Sol-Gel Synthesis of Spherical Lead Titanate Submicrometer Powders. Crystals, 11(5), 484. https://doi.org/10.3390/cryst11050484