Effect of Rheology of Fresh Paste on the Pore Structure and Properties of Pervious Concrete Based on the High Fluidity Alkali-Activated Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Details
2.2.1. Specimen Preparation
2.2.2. Analytical Techniques
3. Results and Discussion
3.1. Workability of Fresh Paste
3.2. Sedimentation of Paste
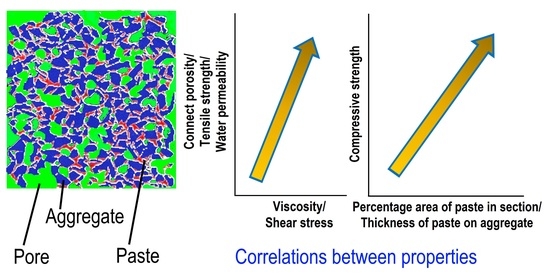
3.3. Mesostructure
3.4. Thickness of Paste on the Surface of Coarse Aggregate
3.5. Strength and Water Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosic, K.; Korat, L.; Ducman, V.; Netinger, I. Influence of aggregate type and size on properties of pervious concrete. Constr. Build. Mater. 2015, 78, 69–76. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Wang, X.; Zhou, W. Strength, fracture and fatigue of pervious concrete. Constr. Build. Mater. 2013, 42, 97–104. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.-J.; Liang, D. Mechanical properties of pervious cement concrete. J. Cent. South Univ. 2012, 19, 3329–3334. [Google Scholar] [CrossRef]
- Sata, V.; Wongsa, A.; Chindaprasirt, P. Properties of pervious geopolymer concrete using recycled aggregates. Constr. Build. Mater. 2013, 42, 33–39. [Google Scholar] [CrossRef]
- Tho-in, T.; Sata, V.; Chindaprasirt, P.; Jaturapitakkul, C. Pervious high-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2012, 30, 366–371. [Google Scholar] [CrossRef]
- Chandrappa, A.K.; Biligiri, K.P. Comprehensive investigation of permeability characteristics of pervious concrete: A hydrodynamic approach. Constr. Build. Mater. 2016, 123, 627–637. [Google Scholar] [CrossRef]
- Kim, Y.J.; Gaddafi, A.; Yoshitake, I. Permeable concrete mixed with various admixtures. Mater. Des. 2016, 100, 110–119. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W.; Fu, T.C.; Huang, R. Properties of pervious concrete made with electric arc furnace slag and alkali-activated slag cement. Constr. Build. Mater. 2016, 109, 34–40. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Sun, B.; Yin, J. Experimental investigation on freeze-thaw durability of Portland cement pervious concrete (PCPC). Constr. Build. Mater. 2016, 117, 63–71. [Google Scholar] [CrossRef]
- Dong, Q.; Wu, H.; Huang, B.; Shu, X.; Wang, K. Investigation into Laboratory Abrasion Test Methods for Pervious Concrete. J. Mater. Civ. Eng. 2013, 25, 886–892. [Google Scholar] [CrossRef]
- Haselbach, L.; Poor, C.; Tilson, J. Dissolved zinc and copper retention from stormwater runoff in ordinary portland cement pervious concrete. Constr. Build. Mater. 2014, 53, 652–657. [Google Scholar] [CrossRef]
- Kim, G.M.; Jang, J.G.; Khalid, H.R.; Lee, H.K. Water purification characteristics of pervious concrete fabricated with CSA cement and bottom ash aggregates. Constr. Build. Mater. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Jo, M.; Soto, L.; Arocho, M.; St John, J.; Hwang, S. Optimum mix design of fly ash geopolymer paste and its use in pervious concrete for removal of fecal coliforms and phosphorus in water. Constr. Build. Mater. 2015, 93, 1097–1104. [Google Scholar] [CrossRef]
- Yeih, W.; Fu, T.C.; Chang, J.J.; Huang, R. Properties of pervious concrete made with air-cooling electric arc furnace slag as aggregates. Constr. Build. Mater. 2015, 93, 737–745. [Google Scholar] [CrossRef]
- Zaetang, Y.; Wongsa, A.; Sata, V.; Chindaprasirt, P. Use of coal ash as geopolymer binder and coarse aggregate in pervious concrete. Constr. Build. Mater. 2015, 96, 289–295. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 1. In situ energy-dispersive X-ray diffractometry. Chem. Eng. Sci. 2007, 62, 2309–2317. [Google Scholar] [CrossRef]
- Provis, J.L.; Walls, P.A.; van Deventer, J.S.J. Geopolymerisation kinetics. 3. Effects of Cs and Sr salts. Chem. Eng. Sci. 2008, 63, 4480–4489. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. In Annual Review of Materials Research; Clarke, D.R., Ed.; Annual Reviews; Palo Alto: Santa Clara, CA, USA, 2014; Volume 44, pp. 299–327. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Alkaline activation of aluminosilicates as an alternative to portland cement: A review. Rev. Romana Mater. Rom. J. Mater. 2017, 47, 3–15. [Google Scholar]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Attwell, C. Geopolymer concrete: A practical approach. Constr. Mater. Struct. 2014, 466–474. [Google Scholar] [CrossRef]
- Majidi, B. Geopolymer technology, from fundamentals to advanced applications: A review. Mater. Technol. 2009, 24, 79–87. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an indexing approach to evaluate fly ashes for geopolymer manufacture. Cem. Concr. Res. 2016, 85, 163–173. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Provis, J.L. Green concrete or red herring?-future of alkali-activated materials. Adv. Appl. Ceram. 2014, 113, 472–477. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C.J. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Navarro, R.; Zornoza, E.; Garces, P.; Sanches, I.; Alcocel, E.G. Optimization of the alkali activation conditions of ground granulated SiMn slag. Constr. Build. Mater. 2017, 150, 781–791. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenovic, M.; Bascarevic, Z.; Nikolic, V.; Petrovic, R. Physical-mechanical and microstructural properties of alkali-activated fly ash-blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Wang, W.C.; Wang, H.Y.; Tsai, H.C. Study on engineering properties of alkali-activated ladle furnace slag geopolymer. Constr. Build. Mater. 2016, 123, 800–805. [Google Scholar] [CrossRef]
- Jang, J.G.; Ahn, Y.B.; Souri, H.; Lee, H.K. A novel eco-friendly porous concrete fabricated with coal ash and geopolymeric binder: Heavy metal leaching characteristics and compressive strength. Constr. Build. Mater. 2015, 79, 173–181. [Google Scholar] [CrossRef]
- GB/T 14685-2011: Pebble and Crushed Stone for Construction; Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- Sun, H. Mix Design and Properties of Pervious Concrete Based on the Optimization Method; Southwest Jiaotong University: Chengdu, China, 2016. [Google Scholar]
- GB/T 2419-2005: Test Method for Fluidity of Cement Mortar; National Standardization Administration Commission: Beijing, China, 2005.
- Torres, A.; Hu, J.; Ramos, A. The effect of the cementitious paste thickness on the performance of pervious concrete. Constr. Build. Mater. 2015, 95, 850–859. [Google Scholar] [CrossRef]
- DB11/T 775-2010: Technical Specification for Pervious Concrete Pavement; Quality Supervision Bureau of Beijing: Beijing, China, 2010.
- International, A. ASTM C1754/C1754M-12: Standard Test Method for Density and Void Content of Hardened Pervious Concrete; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Japanese Concrete Institute. Technical Committee Report on Eco-Concrete; Japanese Concrete Institute: Tokyo, Japan, 1995. [Google Scholar]
- Japanese Concrete Institute. Technical Committee Report on Design and Construction of Porous Concrete; Japanese Concrete Institute: Tokyo, Japan, 2003. [Google Scholar]
- JTG E30-2005: Test Methods of Cement and Concrete for Highway Engineering; Ministry of Transport of the People’s Republic of China: Beijing, China, 2005.
- Dang Hanh, N.; Boutouil, M.; Sebaibi, N.; Leleyter, L.; Baraud, F. Valorization of seashell by-products in pervious concrete pavers. Constr. Build. Mater. 2013, 49, 151–160. [Google Scholar] [CrossRef]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; van Deventer, J.S.J. The interrelationship between surface chemistry and rheology in alkali activated slag paste. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Alkali-activated binders-chemistry and engineering. In Advances in Chemically-Activated Materials; Shi, C., Shen, X., Eds.; RILEM Publications: Bagneux, France, 2014; Volume 92, p. 3. [Google Scholar]
- Ravikumar, D.; Neithalath, N. Effects of activator characteristics on the reaction product formation in slag binders activated using alkali silicate powder and NaOH. Cem. Concr. Compos. 2012, 34, 809–818. [Google Scholar] [CrossRef]
- Beersaerts, G.; Vananroye, A.; Sakellariou, D.; Clasen, C.; Pontikes, Y. Rheology of an alkali-activated Fe-rich slag suspension: Identifying the impact of the activator chemistry and slag particle interactions. J. Non-Cryst. Solids 2021, 561, 120747. [Google Scholar] [CrossRef]
- Aminul Islam, L.; Rajan, B. Rheology of Fly-Ash-Based Geopolymer Concrete. ACI Mater. J. 2011, 108. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gismera, S.; Blanco, M.T.; Lanzón, M.; Puertas, F. Alkali-activated mortars: Workability and rheological behaviour. Constr. Build. Mater. 2017, 145, 576–587. [Google Scholar] [CrossRef]
- Bentz, D.P.; Ferraris, C.F.; Galler, M.A.; Hansen, A.S.; Guynn, J.M. Influence of particle size distributions on yield stress and viscosity of cement–fly ash pastes. Cem. Concr. Res. 2012, 42, 404–409. [Google Scholar] [CrossRef]
- Vance, K.; Dakhane, A.; Sant, G.; Neithalath, N. Observations on the rheological response of alkali activated fly ash suspensions: The role of activator type and concentration. Rheol. Acta 2014, 53, 843–855. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Rimer, J.D.; Lobo, R.F.; Vlachos, D.G. Physical basis for the formation and stability of silica nanoparticles in basic solutions of monovalent cations. Langmuir 2005, 21, 8960–8971. [Google Scholar] [CrossRef]
- Barnhouse, P.W.; Srubar, W.V. Material characterization and hydraulic conductivity modeling of macroporous recycled-aggregate pervious concrete. Constr. Build. Mater. 2016, 110, 89–97. [Google Scholar] [CrossRef]



















| Oxide | CaO | Si2O | Al2O3 | MgO | SO3 | TiO2 | Fe2O3 | Na2O | L.O.I. * |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 38.7 | 35.5 | 14.8 | 6.7 | 2.2 | 0.79 | 0.27 | 0.26 | 0.88 |
| Modulus | Solid Content | Na2O Content | SiO Content | Density | Transparency | Fe Content |
|---|---|---|---|---|---|---|
| 2.2 | 53 wt% | 24.5 wt% | 53.9 wt% | 1495 kg/m3 | 88% | 0.08% |
| Type | Bulk Density | Apparent Density | Crushing Value | Water Absorption | Moisture Content |
|---|---|---|---|---|---|
| Limestone | 1560.5 kg/m3 | 2680 kg/m3 | 4.57% | 3.2% | 0.3% |
| Mix | Slag | Equivalent Na2O Content | NaOH | Water Glass | Retarder | Water | Aggregate | DVR § |
|---|---|---|---|---|---|---|---|---|
| A4 | 305 | 4% | 5.01 | 49.11 | 0.3 | 68.42 | 1529 | 22% |
| A6 | 305 | 6% | 7.51 | 73.66 | 0.3 | 56.88 | 1529 | 21.5% |
| A8 | 305 | 8% | 10.02 | 98.22 | 0.3 | 45.34 | 1529 | 21% |
| A10 | 305 | 10% | 12.52 | 122.77 | 0.3 | 33.80 | 1529 | 20.4% |
| Part | N | Average Thickness (mm) | Thickness Distribution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2–0.6 mm | 0.6–1 mm | 1–1.4 mm | 1.4–1.8 mm | 1.8–2.2 mm | 2.2–2.6 mm | 2.6–3 mm | 3–5 mm | 5–9 mm | ||||
| A4 | Up | 144 | 0.919 | 15% | 19% | 22% | 14% | 11% | 4% | 4% | 1% | 3% |
| Dn | 212 | 0.929 | 16% | 20% | 19% | 18% | 7% | 10% | 3% | 2% | 2% | |
| A6 | Up | 120 | 0.941 | 3% | 27% | 20% | 22% | 8% | 3% | 3% | 5% | 0% |
| Dn | 182 | 1.003 | 8% | 20% | 15% | 29% | 11% | 7% | 3% | 7% | 0% | |
| A8 | Up | 158 | 1.123 | 8% | 22% | 6% | 22% | 9% | 8% | 6% | 15% | 0% |
| Dn | 174 | 1.151 | 15% | 29% | 11% | 13% | 8% | 6% | 5% | 9% | 0% | |
| A10 | Up | 132 | 1.089 | 15% | 17% | 21% | 9% | 6% | 8% | 6% | 11% | 3% |
| Dn | 172 | 0.965 | 10% | 27% | 20% | 15% | 10% | 5% | 2% | 7% | 1% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Xu, Q.; Duraman, S.B.; Li, Q. Effect of Rheology of Fresh Paste on the Pore Structure and Properties of Pervious Concrete Based on the High Fluidity Alkali-Activated Slag. Crystals 2021, 11, 593. https://doi.org/10.3390/cryst11060593
Geng H, Xu Q, Duraman SB, Li Q. Effect of Rheology of Fresh Paste on the Pore Structure and Properties of Pervious Concrete Based on the High Fluidity Alkali-Activated Slag. Crystals. 2021; 11(6):593. https://doi.org/10.3390/cryst11060593
Chicago/Turabian StyleGeng, Haining, Qing Xu, Saiful B. Duraman, and Qiu Li. 2021. "Effect of Rheology of Fresh Paste on the Pore Structure and Properties of Pervious Concrete Based on the High Fluidity Alkali-Activated Slag" Crystals 11, no. 6: 593. https://doi.org/10.3390/cryst11060593
APA StyleGeng, H., Xu, Q., Duraman, S. B., & Li, Q. (2021). Effect of Rheology of Fresh Paste on the Pore Structure and Properties of Pervious Concrete Based on the High Fluidity Alkali-Activated Slag. Crystals, 11(6), 593. https://doi.org/10.3390/cryst11060593