Mechanism and Effect of the Dilution Gas Flow Rate on Various Fe–Si/SiO2 Soft Magnetic Composites during Fluidised Bed Chemical Vapour Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe–Si/SiO2 Composites Using FBCVD-HPS
2.3. Characterisation
3. Results
3.1. Morphology of Fe–Si/SiO2 Composite Particles and Soft Magnetic Composites
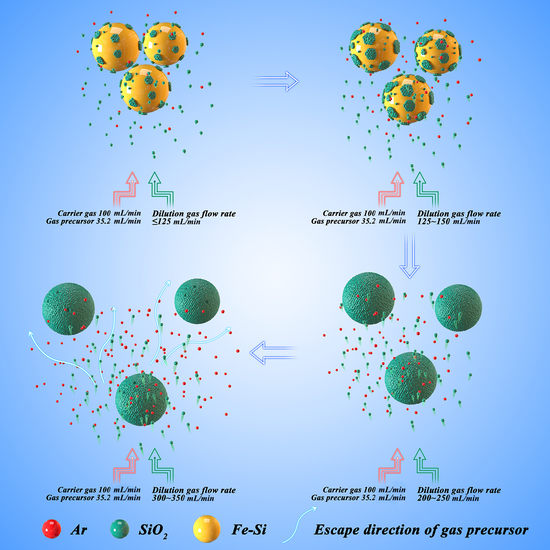
3.2. Influential Mechanism of the Ar-Dilution Gas Flow Rate
- When the Ar dilution gas flow is relatively low (125 mL/min), the dynamic viscosity of Ar gas increases with the reaction temperature, although VAr before the reaction is greater than VFe(Si). The Fe–Si substrate particles cannot continue to maintain fluidisation and the gas SiO2 precursor contacts with the partial surface of accumulated Fe–Si substrate particles. At this stage, the SiO2 deposition presents submicron clusters.
- As the Ar dilution gas flow rate increases to 150 mL/min, the Fe–Si substrate particles break away from the state of being unable to fluidise because of high temperature, and the gas SiO2 precursor passes through the substrate particles. However, the content of the gas SiO2 precursor in the reaction system is less because of the low flow rate of the Ar dilution gas. The submicron SiO2 clusters transition towards incomplete and uneven SiO2 layers. Simultaneously, the deposition rate of the SiO2 layers drops.
- When the Ar-dilution gas flow rate increases to some extent (200 and 250 mL/min), the Ar dilution gas can maintain the Fe–Si substrate particles in a fluidised suspension motion despite the increase in temperature during the deposition reaction. The gaseous SiO2 precursor is uniformly distributed on the surface of the Fe–Si substrate particles. According to the theory of sedimentary process [21], Ar gas functions as a carrier and dilute gas precursor, belonging to quality transport control. The diffusion resistance of the gaseous SiO2 precursor through the gasflow boundary layer decreases with in the increasing Ar-dilution gas flow rate. Thus, the number of gas–solid collisions and the reaction rate at the gas–solid interface are increased by decreasing the occurrence of homogenous gas-nucleation reactions before contact with the Fe–Si substrate particle surface. As the Ar dilution gas flow rate increases, the SiO2 layer deposition rate increases. SiO2 layers exist in the form of a coated film.
- However, as the Ar-dilution gas flow rate continues to increase (300 and 350 mL/min), the excessive Ar dilution gas flow, at this time, reduces the diffusion resistance of the gaseous SiO2 precursor, the retention time of the gaseous SiO2 precursor in the vertical tube furnace, and the effective reaction time with the Fe–Si substrate particle surface. Certain gaseous SiO2 precursors are expelled with the Ar dilution gas prior to deposition, and the high gas flow rate will reduce the temperature around the Fe–Si substrate particles. The deposition rate of the SiO2 layer at this Ar-dilution gas flow rate decreases owing to the reduced reaction rate at the gas–solid interface. Thus, surface defects, such as holes, appear in the SiO2 layers.
3.3. Crystal Structure Evolution during HPS
3.4. Magnetic Behaviour of Fe–Si/SiO2 Soft Magnetic Composites
4. Conclusions
- With an increase in the Ar-dilution gas flow rate from 125 to 350 mL/min during FBCVD, SiO2 deposited on the Fe–Si substrate particle surface presented the microscopic characteristics of “sub-micron SiO2 clusters (125 mL/min) → incomplete SiO2 layers (150 mL/min) → complete SiO2 film (200 and 250 mL/min) → porous SiO2 film (300 and 350 mL/min)”.
- The dynamic disordered structure of beta-cristobalite was similar to the medium-range ordered structure of amorphous SiO2, increasing the reaction rate of the transformation from amorphous SiO2 to beta-cristobalite. Thus, SiO2 layers transformed from the amorphous to the beta-cristobalite state with a hexagonal crystal structure at 1226.05 K during HPS. However, the crystallisation of SiO2 layers did not affect the crystal structure of Fe–Si substrate particles.
- The saturation magnetisation of Fe–Si/SiO2 soft magnetic composites initially decreased and then increased with increasing Ar-dilution gas flow rate. The electrical resistivity of Fe–Si/SiO2 soft magnetic composites slowly increased before 150 mL/min, followed by a rapid increase, between 150 and 250 mL/min, and then decreased while the total core loss exhibited the opposite trend. The lowest total core loss value (215.7 W/kg) was observed at the Ar-dilution gas flow rate of 250 mL/min.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shokrollahi, H.; Janghorban, K. Soft magnetic composite materials (SMCs). J. Mater. Process. Technol. 2007, 189, 1–12. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Thiagarajan, Y.; Rajendirakumar, R.; Basanth, A.J.; Karthikeyan, R.; Kannan, S. Development of an iron powder metallurgy soft magnetic composite core switched reluctance motor. Mater. Today Proc. 2021, 41, 1195–1201. [Google Scholar]
- Talaat, A.; Suraj, M.V.; Byerly, K.; Wang, A.; Wang, Y.; Lee, J.K.; Ohodnicki, P.R. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloys Compd. 2021, 870, 159500. [Google Scholar] [CrossRef]
- Svensson, L.; Frogner, K.; Jeppsson, P.; Cedell, T.; Andersson, M. Soft magnetic moldable composites: Properties and applications. J. Magn. Magn. Mater. 2012, 324, 2717–2722. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, X.; Jian, W.; Li, G.; Zhang, Z. Core loss reduction in Fe–6.5 wt.%Si/SiO2 core–shell composites by ball milling coating and spark plasma sintering. J. Alloys Compd. 2014, 617, 21–28. [Google Scholar] [CrossRef]
- Gheisari, K.; Javadpour, S.; Shokrollahi, H.; Hashemi, B. Magnetic losses of the soft magnetic composites consisting of iron and Ni–Zn ferrite. J. Magn. Magn. Mater. 2008, 320, 1544–1548. [Google Scholar] [CrossRef]
- Clapsaddle, B.J.; Gash, A.E.; Satcher, J.H.; Simpson, R.L. Silicon oxide in an iron(III) oxide matrix: The sol–gel synthesis and characterization of Fe–Si mixed oxide nanocomposites that contain iron oxide as the major phase. J. Non-Cryst. Solids 2003, 331, 190–201. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, Y.; Chi, Q.; Zhang, Y.; Chang, L.; Gong, M.; Huang, J.; Pan, Y.; Wang, X. Fe-based amorphous soft magnetic composites with SiO2 insulation coatings: A study on coatings thickness, microstructure and magnetic properties. Ceram. Int. 2020, 46, 13449–13459. [Google Scholar] [CrossRef]
- Aizawa, T.; Ito, M.; Kihara, J. Acoustic diagnosis for nondestructive evaluation of ceramic coatings on the steel substrates. Am. Soc. Mech. Eng. 1995, 302, 257–270. [Google Scholar]
- Wu, Z.Y.; Jiang, Z.; Fan, X.A.; Zhou, L.J.; Wang, W.L.; Xu, K. Facile synthesis of Fe-6.5wt%Si/SiO2 soft magnetic composites as an efficient soft magnetic composite material at medium and high frequencies. J. Alloys Compd. 2018, 742, 90–98. [Google Scholar] [CrossRef]
- Liu, L.; Liao, X.W.; Jia, J.X.; Kong, H.; Fan, X.A.; Wu, Z.Y.; Wang, X.S. Temperature-controlled conversion from Fe–Si particles to integrated Fe–Si/SiO2 core–shell structure particles during fluidised bed chemical vapour deposition. Ceram. Int. 2020, 46, 3059–3065. [Google Scholar] [CrossRef]
- Labropoulos, A.I.; Romanos, G.E.; Karanikolos, G.N.; Katsaros, F.K.; Kakizis, N.K.; Kanellopoulos, N.K. Comparative study of the rate and locality of silica deposition during the CVD treatment of porous membranes with TEOS and TMOS. Microporous Mesoporous Mater. 2009, 120, 177–185. [Google Scholar] [CrossRef]
- Baux, A.; Jacques, S.; Allemand, A.; Vignoles, G.L.; David, P.; Piquero, T.; Stempin, M.P.; Chollon, G. Complex geometry macroporous SiC ceramics obtained by 3D-printing, polymer impregnation and pyrolysis (PIP) and chemical vapor deposition (CVD). J. Eur. Ceram. Soc. 2021, 41, 3274–3284. [Google Scholar] [CrossRef]
- Bain, M.F.; Low, Y.H.; Bien, D.C.S.; Montgomery, J.H.; Armstrong, B.M.; Gamble, H.S. Investigation into the selectivity of CVD iron from Fe(CO)5 precursor on various metal and dielectric patterned substrates. Surf. Coat. Technol. 2007, 201, 8998–9002. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Zubkov, S.V.; Shuvaeva, V.A.; Abdulvakhidov, K.G.; Shevtsova, S.I. Crystal structure and dielectric properties of aurivillius phases A 0.5 Bi 4.5 B 0.5 Ti 3.5 O 15 ( A = Na, Ca, Sr, Pb; B = Cr, Co, Ni, Fe, Mn, Ga). Physics Solid State 2014, 56, 1554–1560. [Google Scholar] [CrossRef]
- Zhang, S.; Hattori, N.; Enomoto, M.; Tarui, T. Ferrite Nucleation at Ceramic/Austenite Interfaces. ISIJ Int. 1996, 36, 1301–1309. [Google Scholar] [CrossRef]
- Bertrand, N.; Maury, F.; Duverneuil, P. SnO2 coated Ni particles prepared by fluidized bed chemical vapor deposition. Surf. Coat. Technol. 2006, 200, 6733–6739. [Google Scholar] [CrossRef] [Green Version]
- Cadoret, L.; Rossignol, C.; Dexpert-Ghys, J.; Caussat, B. Chemical vapor deposition of silicon nanodots on TiO2 submicronic powders in vibrated fluidized bed. Mater. Sci. Eng. B 2010, 170, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, A.; Lu, J.; Zhang, Z.; He, H.; Li, C. In situ synthesis of CNTs/Fe–Ni/TiO2 nanocomposite by fluidized bed chemical vapor deposition and the synergistic effect in photocatalysis. Particuology 2014, 14, 24–32. [Google Scholar] [CrossRef]
- Amarasinghe, W.S.; Jayarathna, C.K.; Ahangama, B.S.; Tokheim, L.A.; Moldestad, B.M.E. Experimental Study and CFD Modelling of Minimum Fluidization Velocity for Geldart A, B and D Particles. Int. J. Modeling Optim. 2017, 7, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Grabke, H.J.; Moszynski, D.; Müller-Lorenz, E.; Schneider, A. Role on sulfur in carburization, carbide formation and metal dusting on iron. Surf. Interface Anal. 2010, 34, 369–374. [Google Scholar] [CrossRef]
- Frontera, C.; Rodríguez-Carvajal, J. FullProf as a new tool for flipping ratio analysis: Further improvements. Phys. B Condens. Matter 2004, 350, E731–E733. [Google Scholar] [CrossRef]
- Nordeng, S.H.; Sibley, D.F. Dolomite stoichiometry and Ostwald’s Step Rule. Geochim. Cosmochim. Acta 1994, 58, 191–196. [Google Scholar] [CrossRef]
- Morse, J.W.; Casey, W.H. Ostwald processes and mineral paragenesis in sediments. Am. J. Sci. 1988, 288, 537–560. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-Consistent Thermodynamic Data for Minerals in the System Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]







| Refinement Parameters | Phase | Cell Parameters | Bond Angle | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rwp | Rp | S | a | b | c | α | β | γ | ||
| Fe–Si/SiO2 composite particles | 5.58% | 4.18% | 2.36 | Fe–Si | 2.859 | 2.859 | 2.859 | 90.00 | 90.00 | 90.00 |
| Fe–Si/SiO2 soft magnetic composites | 5.96% | 4.37% | 2.78 | Fe–Si | 2.859 | 2.859 | 2.859 | 90.00 | 90.00 | 90.00 |
| SiO2 | 5.041 | 5.041 | 5.253 | 90.00 | 90.00 | 120.00 | ||||
| Samples | Ar Dilution Gas Flow Rate (mL/min) | Total Core Loss (W/kg) | ||||
|---|---|---|---|---|---|---|
| W10/25 | W10/50 | W10/100 | W10/150 | W10/200 | ||
| Fe–Si compacts | 0 | 19.8 | 54.6 | 151.4 | 274.1 | 423.2 |
| Fe–Si/SiO2 composite compacts | 125 | 19.5 | 53.9 | 150.7 | 225.5 | 275.8 |
| 150 | 15.6 | 42.8 | 119 | 215.9 | 271.2 | |
| 200 | 13.9 | 39.1 | 108.1 | 197.4 | 259.3 | |
| 250 | 11.0 | 30.7 | 83.7 | 152.7 | 215.7 | |
| 300 | 11.4 | 31.9 | 90.6 | 169.5 | 221.5 | |
| 350 | 13.9 | 38.3 | 106.4 | 192.1 | 232.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Gao, Z.; Zhao, Q.; Kong, H.; Li, M.; Jia, J. Mechanism and Effect of the Dilution Gas Flow Rate on Various Fe–Si/SiO2 Soft Magnetic Composites during Fluidised Bed Chemical Vapour Deposition. Crystals 2021, 11, 963. https://doi.org/10.3390/cryst11080963
Wu Z, Gao Z, Zhao Q, Kong H, Li M, Jia J. Mechanism and Effect of the Dilution Gas Flow Rate on Various Fe–Si/SiO2 Soft Magnetic Composites during Fluidised Bed Chemical Vapour Deposition. Crystals. 2021; 11(8):963. https://doi.org/10.3390/cryst11080963
Chicago/Turabian StyleWu, Zhaoyang, Zihan Gao, Qian Zhao, Hui Kong, Mingyang Li, and Jixiang Jia. 2021. "Mechanism and Effect of the Dilution Gas Flow Rate on Various Fe–Si/SiO2 Soft Magnetic Composites during Fluidised Bed Chemical Vapour Deposition" Crystals 11, no. 8: 963. https://doi.org/10.3390/cryst11080963
APA StyleWu, Z., Gao, Z., Zhao, Q., Kong, H., Li, M., & Jia, J. (2021). Mechanism and Effect of the Dilution Gas Flow Rate on Various Fe–Si/SiO2 Soft Magnetic Composites during Fluidised Bed Chemical Vapour Deposition. Crystals, 11(8), 963. https://doi.org/10.3390/cryst11080963