Novel NiMgOH-rGO-Based Nanostructured Hybrids for Electrochemical Energy Storage Supercapacitor Applications: Effect of Reducing Agents
Abstract
:1. Introduction
2. Materials and Methods
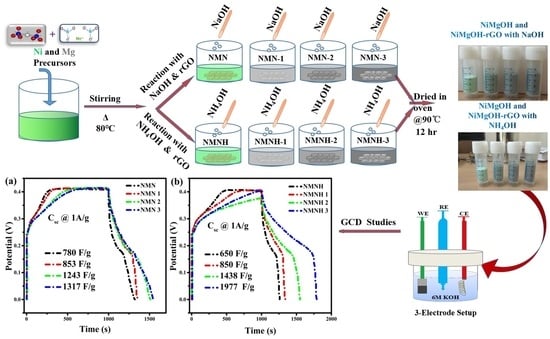
2.1. Synthesis of NiMgOH and NiMgOH-rGO Using Co-Precipitation Method with NaOH and NH4OHas Reducing Agents
2.2. Characterization
3. Results and Discussions
3.1. X-ray Diffraction (XRD) Studies
3.2. Particle Size Analysis (PSA)
3.3. Fourier-Transform Infrared Spectroscopic Studies
3.4. UV-Visible Spectroscopy
3.5. Electrochemical Studies
3.5.1. Electrode Preparation
3.5.2. Cyclic Voltammetry Studies
3.5.3. Galvanostatic Charge–Discharge Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, C.V.; Reddy, I.N.; Reddy, K.R.; Jaesool, S.; Yoo, K. Template-free synthesis of tetragonal Co-doped ZrO2 nanoparticles for applications in electrochemical energy storage and water treatment. Electrochim. Acta 2019, 317, 416–426. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Shinde, P.A.; Patil, S.J.; Hwang, S.K.; Raju, G.S.R.; Ranjith, K.S.; Dubal, D.P.; Huh, Y.S.; Han, Y.K. Solution-free self-assembled growth of ordered tricopper phosphide for efficient and stable hybrid supercapacitor. Energy Storage Mater. 2021, 39, 194–202. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Pawar, K.; Chavan, U.S. Chemically deposited ultrathin α-Ni(OH)2 nanosheet using surfactant on Ni foam for high performance supercapacitor application. Mater. Sci. Energy Technol. 2018, 1, 166–170. [Google Scholar] [CrossRef]
- Edition, E. New supercapacitor for faster electric vehicles. Auto Technol. 2020, 15, 1–4. [Google Scholar]
- Liang, J.; Zhao, Y.; Guo, L.; Li, L. Flexible free-standing graphene/SnO2 nanocomposites paper for Li-ion battery. ACS Appl. Mater. Interfaces 2012, 4, 5742–5748. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, K.; Zhang, R.; Yang, Y.; Wu, Y.; Yu, P. Si Quantum dots assist synthesized microflower-like Si/MoS2 composites for supercapacitors. Crystals 2020, 10, 846. [Google Scholar] [CrossRef]
- Phul, R.; Khan, M.A.M.; Sardar, M.; Ahmed, J.; Ahmad, T. Multifunctional electrochemical properties of synthesized non-precious iron oxide nanostructures. Crystals 2020, 10, 751. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Bagal, I.V.; Ryu, S.W.; Kim, D.H. Hybrid material passivation approach to stabilize the silicon nanowires in aqueous electrolyte for high-energy efficient supercapacitor. Chem. Eng. J. 2019, 362, 609–618. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Chavan, U.S. Nanostructured Ni(OH)2/rGO composite chemically deposited on Ni foam for high performance of supercapacitor applications. Mater. Sci. Energy Technol. 2019, 2, 52–56. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Z.; Liu, Z.; Zhao, Y.; Li, M.; Meng, J.; Tian, X.; Xu, X.; Mai, L. Recent advances in nanowire-based, flexible, freestanding electrodes for energy storage. Chem.-A Eur. J. 2018, 24, 18307–18321. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, Y.; Zhu, R.; Wang, Q.; Luo, Y.; Zhu, C.; Lyu, Y. Rapid synthesis of Ni(OH)2/graphene nanosheets and NiO@Ni(OH)2/graphene nanosheets for supercapacitor applications. New J. Chem. 2019, 43, 3091–3098. [Google Scholar] [CrossRef]
- Chou, S.; Wang, J.; Chew, S.; Liu, H.; Dou, S. Electrochemistry communications electrodeposition of MnO2 nanowires on carbon nanotube paper as free-standing, flexible electrode for supercapacitors. Electrochem. Commun. 2008, 10, 1724–1727. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Li, X.; Guo, H.; Zhang, D.; Wang, Z. Smartly tailored Co(OH)2-Ni(OH)2 heterostructure on nickel foam as binder-free electrode for high-energy hybrid capacitors. Electrochim. Acta 2019, 309, 140–147. [Google Scholar] [CrossRef]
- Ji, S.H.; Chodankar, N.R.; Jang, W.S.; Kim, D.H. High mass loading of h-WO3 and α-MnO2 on flexible carbon cloth for high-energy aqueous asymmetric supercapacitor. Electrochim. Acta 2019, 299, 245–252. [Google Scholar] [CrossRef]
- Jablonskiene, J.; Simkunaite, D.; Vaiciuniene, J.; Stalnionis, G.; Drabavicius, A.; Jasulaitiene, V.; Pakstas, V.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Synthesis of carbon-supported mno2 nanocomposites for supercapacitors application. Crystals 2021, 11, 784. [Google Scholar] [CrossRef]
- Liang, T.; Xuan, H.; Xu, Y.; Gao, J.; Han, X.; Yang, J.; Han, P.; Wang, D.; Du, Y. Rational Assembly of CoAl-Layered Double Hydroxide on Reduced Graphene Oxide with Enhanced Electrochemical Performance for Energy Storage. ChemElectroChem 2018, 5, 2424–2434. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, G.; Gao, X.; Chen, J.; Zhang, L.; Xu, J. α -and β -Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials 2019, 9, 1686. [Google Scholar] [CrossRef] [Green Version]
- Memon, J.; Sun, J.; Meng, D.; Ouyang, W.; Memon, M.A.; Huang, Y.; Yan, S.; Geng, J. Synthesis of graphene/Ni-Al layered double hydroxide nanowires and their application as an electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 5060–5067. [Google Scholar] [CrossRef]
- Vidotti, M.; Salvador, R.P.; Ponzio, E.A.; de Torresi, S.I.C. Mixed Ni/Co hydroxide nanoparticles synthesized by sonochemical method. J. Nanosci. Nanotechnol. 2007, 7, 3221–3226. [Google Scholar] [CrossRef]
- Li, X.; Khalafallah, D.; Wu, Z.; Zhi, M.; Hong, Z. Silver incorporated partially reduced NiCo-layered double hydroxide frameworks for asymmetric supercapacitors. J. Energy Storage 2020, 31, 101578. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Lou, G.; Wu, Y.; Zhu, X.; Chen, H.; Shen, Z.; Fu, S.; Bao, B.; Wu, L. Synthesis of NiMn-LDH nanosheet@Ni3S2 nanorod hybrid structures for supercapacitor electrode materials with ultrahigh specific capacitance. Sci. Rep. 2018, 8, 5246. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P.; Wang, Y.; Li, J. Preparation of Mg(OH)2 nanosheets and self-assembly of its flower-like nanostructure via precipitation method for heat-resistance application. Integr. Ferroelectr. 2015, 163, 148–154. [Google Scholar] [CrossRef]
- Stephen, A. Protective Coatings for Magnesium Alloys; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Song, G.; StJohn, D. Corrosion behaviour of magnesium in ethylene glycol. Corros. Sci. 2004, 46, 1381–1399. [Google Scholar] [CrossRef]
- Kang, J.; Schwendeman, S.P. Comparison of the effects of Mg(OH)2 and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D,L-lactide-co-glycolide) implants. Biomaterials 2002, 23, 239–245. [Google Scholar] [CrossRef]
- Zaman, N.; Malik, R.A.; Alrobei, H.; Kim, J.; Latif, M.; Hussain, A.; Maqbool, A.; Karim, R.A.; Saleem, M.; Rafiq, M.A.; et al. Structural and electrochemical analysis of decarburized graphene electrodes for supercapacitor applications. Crystals 2020, 10, 1043. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Abdel-Galeil, M.M.; Matsuda, A. One-pot synthesis of reduced graphene oxide nanosheets anchored ZnO nanoparticles via microwave approach for electrochemical performance as supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2020, 31, 15456. [Google Scholar] [CrossRef]
- Goswami, M.; Saraf, M.; Singh, B.; Mobin, S.M. Physicochemical and electrochemical behaviours of manganese oxide electrodes for supercapacitor application. J. Energy Storage 2020, 28, 101228. [Google Scholar]
- Li, Y.; Song, Q.; Fan, B.; Zhang, R. Effects of reducing agents on the synthesis of Ag/rGO nanocomposites. Mater. Res. Express 2017, 4, 015014. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- De Cremoux, B.; Hirtz, P.; Ricciardi, J. Comment on “miscibility gaps in quaternary III/V alloys”. J. Cryst. Growth 1983, 61, 177–178. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Wainstein, D.L.; Rashkovskiy, A.Y.; Gago, R.; Soldera, F.; Endrino, J.L.; Fox-Rabinovich, G.S. Interface-induced plasmon nonhomogeneity in nanostructured metal-dielectric planar metamaterial. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron (III) Oxides from Thermal Processes s Synthesis. Chem. Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Kulasekarapandian, K.; Jayanthi, S.; Muthukumari, A.; Arulsankar, A.; Sundaresan, B. Preparation and Characterization of PVC-PEO Based Polymer Blend Electrolytes Complexed With Lithium Perchlorate. Int. J. Eng. Res. Dev. 2013, 5, 30–39. [Google Scholar]
- Li, Y.; Yi, R.; Yan, A.; Deng, L.; Zhou, K.; Liu, X. Facile synthesis and properties of ZnFe2O4 and ZnFe2O4/polypyrrole core-shell nanoparticles. Solid State Sci. 2009, 11, 1319–1324. [Google Scholar] [CrossRef]
- Mazen, S.A.; Ahmed, M.A.; Sabrah, B.A. Thermal studies on the electrical conduction mechanism of CuFe2O4. Thermochim. Acta 1982, 56, 229–233. [Google Scholar] [CrossRef]
- Babar, P.T.; Pawar, B.S.; Ahmed, A.T.A.; Sekar, S.; Lee, S.; Sankapal, B.R.; Im, H.; Kim, J.H.; Pawar, S.M. Synthesis of nickel hydroxide/reduced graphene oxide composite thin films for water splitting application. Int. J. Energy Res. 2020, 44, 10908–10916. [Google Scholar] [CrossRef]
- <monospace>Huang, E.-W.; Hung, G.-Y.; Lee, S.Y.; Jain, J.; Chang, K.-P.; Chou, J.J.; Yang, W.-C.; Liaw, P.K. Mechanical and Magnetic Properties of the High-Entropy Alloys for Combinatorial Approaches. Crystals 2020, 10, 200. [Google Scholar]
- Cui, H.; Xue, J.; Ren, W.; Wang, M. Ultra-high specific capacitance of β-Ni(OH)2monolayer nanosheets synthesized by an exfoliation-free sol-gel route. J. Nanoparticle Res. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Elshahawy, A.M.; Ho, K.H.; Hu, Y.; Fan, Z.; Hsu, Y.W.B.; Guan, C.; Ke, Q.; Wang, J. Microwave-assisted hydrothermal synthesis of nanocrystal β-Ni(OH)2 for supercapacitor applications. CrystEngComm 2016, 18, 3256–3264. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, R.; Khan, S. Effect of reduced graphene oxide (rGO) on structural, optical, and dielectric properties of Mg(OH)2/rGO nanocomposites. Adv. Powder Technol. 2017, 28, 2812–2819. [Google Scholar] [CrossRef]
- Halder, M.; Islam, M.M.; Singh, P.; Singha Roy, A.; Islam, S.M.; Sen, K. Sustainable Generation of Ni(OH)2 Nanoparticles for the Green Synthesis of 5-Substituted 1 H-Tetrazoles: A Competent Turn on Fluorescence Sensing of H2O2. ACS Omega 2018, 3, 8169–8180. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.; He, X.; Godwin, I.J.; Backes, C.; McAteer, D.; Berner, N.C.; McEvoy, N.; Ferguson, A.; Shmeliov, A.; Lyons, M.E.G.; et al. Production of Ni(OH)2 nanosheets by liquid phase exfoliation: From optical properties to electrochemical applications. J. Mater. Chem. A 2016, 4, 11046–11059. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Duan, S.; Shen, Y.; Fang, K.; Wang, Y.; Lin, M.; Guo, X. In-Situ-Grown Mg(OH)2-Derived Hybrid α-Ni(OH)2 for Highly Stable Supercapacitor. ACS Energy Lett. 2016, 1, 814–819. [Google Scholar] [CrossRef]
- Zheng, J.H.; Zhang, R.M.; Wang, X.G.; Yu, P.F. Synthesizing a flower-like NiO and ZnO composite for supercapacitor applications. Res. Chem. Intermed. 2018, 44, 5569–5582. [Google Scholar] [CrossRef]
- Cheng, R.; Hu, T.; Zhang, H.; Wang, C.; Hu, M.; Yang, J.; Cui, C.; Guang, T.; Li, C.; Shi, C.; et al. Understanding the lithium storage mechanism of Ti3C2TxMXene. J. Phys. Chem. C 2019, 123, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; John Whiley and Sons: Hoboken, NJ, USA, 2015; pp. 1–25. [Google Scholar]
- Su, W.; Lin, T.; Chu, W.; Zhu, Y.; Li, J.; Zhao, X. Novel synthesis of RGO/NiCoAl-LDH nanosheets on nickel foam for supercapacitors with high capacitance. RSC Adv. 2016, 6, 113123–113131. [Google Scholar] [CrossRef]
- Patil, S.J.; Lokhande, V.C.; Chodankar, N.R.; Lokhande, C.D. Chemically prepared La2Se3 nanocubes thin film for supercapacitor application. J. Colloid Interface Sci. 2016, 469, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.; Plett, G.L. Reduced-order physics-based modeling and experimental parameter identification for non-Faradaic electrical double-layer capacitors. J. Energy Storage 2016, 7, 167–180. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, F.; Li, Y.; Qin, C.; Zhu, J.; Hu, Q.; Wang, Z.; Inoue, A. Flexible NiO micro-rods/nanoporous Ni/metallic glass electrode with sandwich structure for high performance supercapacitors. Electrochim. Acta 2019, 297, 767–777. [Google Scholar] [CrossRef]
- Palagonia, M.S.; Erinmwingbovo, C.; Brogioli, D.; La Mantia, F. Comparison between cyclic voltammetry and differential charge plots from galvanostatic cycling. J. Electroanal. Chem. 2019, 847, 113170. [Google Scholar] [CrossRef]
- Lämmel, C.; Schneider, M.; Weiser, M.; Michaelis, A. Investigations of electrochemical double layer capacitor (EDLC) materials-A comparison of test methods. Materwiss. Werksttech. 2013, 44, 641–649. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Yan, X. Synergistic effect between ultra-small nickel hydroxide nanoparticles and reduced graphene oxide sheets for the application in high-performance asymmetric supercapacitor. Sci. Rep. 2015, 5, 11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Xu, Z.; Duan, S.; Tian, Z.; Zhang, Y.; Xiang, K.; Lin, M.; Guo, X.; Ding, W. Facile growth of homogeneous Ni(OH)2 coating on carbon nanosheets for high-performance asymmetric supercapacitor applications. Nano Res. 2018, 11, 216–224. [Google Scholar] [CrossRef]
- Divya, V.; Mondal, S.; Sangaranarayanan, M. V Shape-Controlled Synthesis of Palladium Nanostructures from Flowers to Thorns: Electrocatalytic Oxidation of Ethanol. J. Nanosci. Nanotechnol. 2018, 19, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Alarco, J.A.; Mackinnon, I.D.R.; Page, D.; Ilyushechkin, A. Synthesis and characterisation of nanoscale magnesium oxide powders and their application in thick films of Bi2Sr2CaCu2O8. Mater. Lett. 1998, 34, 133–142. [Google Scholar] [CrossRef]








| Samples Prepared | Reducing Agents | |
|---|---|---|
| NaOH | NH4OH | |
| NiMgOH | NMN | NMNH |
| NiMgOH-0.5% rGO | NMN-1 | NMNH-1 |
| NiMgOH-1% rGO | NMN-2 | NMNH-2 |
| NiMgOH-1.5% rGO | NMN-3 | NMNH-3 |
| S.No | Name of Sample | Average Particle Size from PSA (nm) | Average Crystallite Size from XRD (nm) |
|---|---|---|---|
| 1 | NMN | 47 | 35 |
| 2 | NMN-1 | 34 | 19 |
| 3 | NMN-2 | 21 | 16 |
| 4 | NMN-3 | 16 | 13 |
| 5 | NMNH | 33 | 23 |
| 6 | NMNH-1 | 29 | 19 |
| 7 | NMNH-2 | 26 | 16 |
| 8 | NMNH-3 | 22 | 15 |
| S.No | Name of Sample | Specific Capacitance (F/g) @ 2 mV/s | Specific Capacitance from GCD Curves @ 1 A/g |
|---|---|---|---|
| 1 | NMN | 568 | 780 |
| 2 | NMN-1 | 733 | 853 |
| 3 | NMN-2 | 809 | 1243 |
| 4 | NMN-3 | 874 | 1317 |
| 5 | NMNH | 787 | 650 |
| 6 | NMNH-1 | 984 | 850 |
| 7 | NMNH-2 | 1170 | 1483 |
| 8 | NMNH-3 | 1793 | 1977 |
| Electrode | RS (Ω) | Rct (Ω) | CPE1 | W | |
|---|---|---|---|---|---|
| Exponent n | Y0 (mΩ−1·sn) | (Ω−1·s1/2) | |||
| NMN | 1.0 | 600 | 0.90 | 0.1 | 0.100 |
| NMN-1 | 1.1 | 400 | 0.95 | 0.5 | 0.010 |
| NMN-2 | 0.91 | 58 | 0.85 | 2.1 | 0.008 |
| NMN-3 | 3.36 | 50 | 0.50 | 1.1 | 0.001 |
| NMNH | 1.15 | 7.42 | 0.50 | 471.4 | 0.197 |
| NMNH-1 | 1.13 | 5.6 | 0.60 | 1.0 | 0.05 |
| NMNH-2 | 1.27 | 2.4 | 0.70 | 80.0 | 0.001 |
| NMNH-3 | 1.32 | 2.0 | 0.95 | 1.0 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shireesha, K.; Kumar, T.R.; Rajani, T.; Chakra, C.S.; Kumari, M.M.; Divya, V.; Raghava Reddy, K. Novel NiMgOH-rGO-Based Nanostructured Hybrids for Electrochemical Energy Storage Supercapacitor Applications: Effect of Reducing Agents. Crystals 2021, 11, 1144. https://doi.org/10.3390/cryst11091144
Shireesha K, Kumar TR, Rajani T, Chakra CS, Kumari MM, Divya V, Raghava Reddy K. Novel NiMgOH-rGO-Based Nanostructured Hybrids for Electrochemical Energy Storage Supercapacitor Applications: Effect of Reducing Agents. Crystals. 2021; 11(9):1144. https://doi.org/10.3390/cryst11091144
Chicago/Turabian StyleShireesha, Konda, Thida Rakesh Kumar, Tumarada Rajani, Chidurala Shilpa Chakra, Murikinati Mamatha Kumari, Velpula Divya, and Kakarla Raghava Reddy. 2021. "Novel NiMgOH-rGO-Based Nanostructured Hybrids for Electrochemical Energy Storage Supercapacitor Applications: Effect of Reducing Agents" Crystals 11, no. 9: 1144. https://doi.org/10.3390/cryst11091144
APA StyleShireesha, K., Kumar, T. R., Rajani, T., Chakra, C. S., Kumari, M. M., Divya, V., & Raghava Reddy, K. (2021). Novel NiMgOH-rGO-Based Nanostructured Hybrids for Electrochemical Energy Storage Supercapacitor Applications: Effect of Reducing Agents. Crystals, 11(9), 1144. https://doi.org/10.3390/cryst11091144