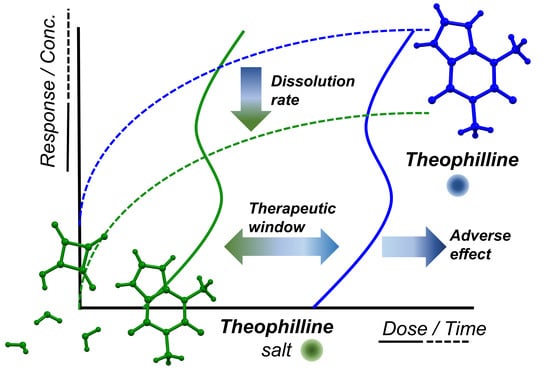
Salification Controls the In-Vitro Release of Theophylline
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Theophylline Squarate
2.3. Single-Crystal X-ray Diffraction (SC-XRD)
2.4. X-ray Powder Diffraction (XRPD)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Thermogravimetric Analysis (TGA)
2.7. Dynamic Vapour Sorption (DVS)
2.8. Dissolution Studies
3. Results
3.1. Theophylline Squarate
3.1.1. Single Crystal Molecular Structure
3.1.2. Thermal and Structural Characterization
3.2. Dissolution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Focaroli, S.; Jiang, G.; O’connell, P.; Fahy, J.V.; Healy, A.M. The Use of a Three-Fluid Atomising Nozzle in the Production of Spray-Dried Theophylline/Salbutamol Sulphate Powders Intended for Pulmonary Delivery. Pharmaceutics 2020, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline: New Perspectives for an Old Drug. Am. J. Respir. Crit. Care Med. 2003, 167, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.Z.; Lin, K.X.; Qian, G.S.; Zhuo, W.L.; Li, S.P.; Zhao, Z.Q.; Liao, X.Q.; Song, Y.X. Comparison of Inhaled Corticosteroid Combined with Theophylline and Double-Dose Inhaled Corticosteroid in Moderate to Severe Asthma. Respirology 2005, 10, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Svedmyr, K. Effects of Oral Theophylline Combined with Oral and Inhaled Β2-Adrenostimulants in Asthmatics. Allergy 1982, 37, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pezoa, R.; Gai, M.N.; Gutierrez, C.; Arancibia, A. Development of a Controlled-Release Theophylline Tablet: Evaluation in Vitro and in Vivo. An. Real Acad. Farm. Inst. Espana 1992, 58, 269–283. [Google Scholar]
- Rodrigues, M.; Peiriço, N.; Matos, H.; Gomes De Azevedo, E.; Lobato, M.R.; Almeida, A.J. Microcomposites Theophylline/Hydrogenated Palm Oil from a PGSS Process for Controlled Drug Delivery Systems. J. Supercrit. Fluids 2004, 29, 175–184. [Google Scholar] [CrossRef]
- Jian, H.; Zhu, L.; Zhang, W.; Sun, D.; Jiang, J. Galactomannan (from Gleditsia Sinensis Lam.) and Xanthan Gum Matrix Tablets for Controlled Delivery of Theophylline: In Vitro Drug Release and Swelling Behavior. Carbohydr. Polym. 2012, 87, 2176–2182. [Google Scholar] [CrossRef]
- Buhecha, M.D.; Lansley, A.B.; Somavarapu, S.; Pannala, A.S. Development and Characterization of PLA Nanoparticles for Pulmonary Drug Delivery: Co-Encapsulation of Theophylline and Budesonide, a Hydrophilic and Lipophilic Drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101128. [Google Scholar] [CrossRef]
- Hayashi, T.; Kanbe, H.; Okada, M.; Suzuki, M.; Ikeda, Y.; Onuki, Y.; Kaneko, T.; Sonobe, T. Formulation Study and Drug Release Mechanism of a New Theophylline Sustained-Release Preparation. Int. J. Pharm. 2005, 304, 91–101. [Google Scholar] [CrossRef]
- Vranić, E. Basic Principles of Drug—Excipients Interactions. Bosn. J. Basic Med. Sci. 2004, 4, 56–58. [Google Scholar] [CrossRef] [PubMed]
- West, R. History of the Oxocarbons, 1st ed.; Academic Press: New York, NY, USA, 1980; pp. 1–14. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tokunaga, Y.; Giovannetti, G.; Picozzi, S.; Itoh, H.; Shimano, R.; Kumai, R.; Tokura, Y. Above-Room-Temperature Ferroelectricity in a Single-Component Molecular Crystal. Nature 2010, 463, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine Dyes: A Mine of Molecular Materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Chemistry of Squaraine-Derived Materials: Near-IR Dyes, Low Band Gap Systems, and Cation Sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Palli, M.A.; McTavish, H.; Kimball, A.; Horn, T.D. Immunotherapy of Recurrent Herpes Labialis with Squaric Acid. JAMA Dermatol. 2017, 153, 828–829. [Google Scholar] [CrossRef]
- Chasák, J.; Šlachtová, V.; Urban, M.; Brulíková, L. Squaric Acid Analogues in Medicinal Chemistry. Eur. J. Med. Chem. 2021, 209, 112872. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.M.; Howard, L.O. Aqueous Dissociation of Squaric Acid. J. Phys. Chem. 1970, 74, 4374–4377. [Google Scholar] [CrossRef]
- Karle, I.L.; Ranganathan, D.; Haridas, V. A Persistent Preference for Layer Motifs in Self-Assemblies of Squarates and Hydrogen Squarates by Hydrogen Bonding [X-H⋯O; X=N, O, or C]: A Crystallographic Study of Five Organic Salts. J. Am. Chem. Soc. 1996, 118, 7128–7133. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. General Rules for the Packing of Hydrogen-Bonded Crystals as Derived from the Analysis of Squaric Acid Anions: Aminoaromatic Nitrogen Base Co-Crystals. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 591–598. [Google Scholar] [CrossRef]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical Hydrates Analysis—Overview of Methods and Recent Advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buist, A.R.; Kennedy, A.R.; Manzie, C. Four Salt Phases of Theophylline. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; Byard, S.J.; Schroeder, S.L.M. Salt or Co-Crystal? Determination of Protonation State by X-Ray Photoelectron Spectroscopy (XPS). J. Pharm. Sci. 2010, 99, 4453–4457. [Google Scholar] [CrossRef] [PubMed]
- Mary Novena, L.; Suresh Kumar, S.; Athimoolam, S. Improved Solubility and Bioactivity of Theophylline (a Bronchodilator Drug) through Its New Nitrate Salt Analysed by Experimental and Theoretical Approaches. J. Mol. Struct. 2016, 1116, 45–55. [Google Scholar] [CrossRef]
- Sarma, B.; Saikia, B. Hydrogen Bond Synthon Competition in the Stabilization of Theophylline Cocrystals. CrystEngComm 2014, 16, 4753–4765. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS-2008/1—Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Allen, F.H.; Cruz-Cabeza, A.J.; Wood, P.A.; Bardwell, D.A. Hydrogen-Bond Landscapes, Geometry and Energetics of Squaric Acid and Its Mono- and Dianions: A Cambridge Structural Database, IsoStar and Computational Study. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 69, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Yang, P.; Liu, Y.; Wang, J.; Gao, Z.; Gong, J.; Rohani, S. Ultrasound-Assisted Theophylline Polymorphic Transformation: Selective Polymorph Nucleation, Molecular Mechanism and Kinetics Analysis. Ultrason. Sonochem. 2021, 77, 105675. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraldi, L.; Fornasari, L.; Bassanetti, I.; Amadei, F.; Bacchi, A.; Marchiò, L. Salification Controls the In-Vitro Release of Theophylline. Crystals 2022, 12, 201. https://doi.org/10.3390/cryst12020201
Baraldi L, Fornasari L, Bassanetti I, Amadei F, Bacchi A, Marchiò L. Salification Controls the In-Vitro Release of Theophylline. Crystals. 2022; 12(2):201. https://doi.org/10.3390/cryst12020201
Chicago/Turabian StyleBaraldi, Laura, Luca Fornasari, Irene Bassanetti, Francesco Amadei, Alessia Bacchi, and Luciano Marchiò. 2022. "Salification Controls the In-Vitro Release of Theophylline" Crystals 12, no. 2: 201. https://doi.org/10.3390/cryst12020201
APA StyleBaraldi, L., Fornasari, L., Bassanetti, I., Amadei, F., Bacchi, A., & Marchiò, L. (2022). Salification Controls the In-Vitro Release of Theophylline. Crystals, 12(2), 201. https://doi.org/10.3390/cryst12020201