A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps
Abstract
:1. Introduction
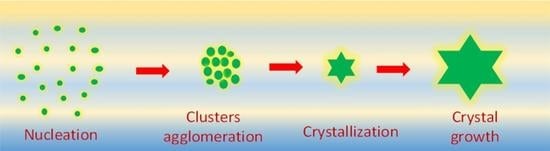
2. Nucleation Mechanism
3. Growth Mechanism
4. Types of Structural Defects in Zeolites
5. Recent Advances in the Modeling of Zeolite Crystallization Using Machine Learning
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asgar Pour, Z.; Sebakhy, K.O. A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry 2022, 4, 431–446. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Abduljawad, M.M.; Alassmy, Y.A.; Cardon, L.; Van Steenberge, P.H.; Sebakhy, K.O. A Comparative Review of Binder-Containing Extrusion and Alternative Shaping Techniques for Structuring of Zeolites into Different Geometrical Bodies. Catalysts 2023, 13, 656. [Google Scholar] [CrossRef]
- Pilar, R.; Moravkova, J.; Sadovska, G.; Sklenak, S.; Brabec, L.; Pastvova, J.; Sazama, P. Controlling the competitive growth of zeolite phases without using an organic structure-directing agent. Synthesis of Al-rich* BEA. Microporous Mesoporous Mater. 2022, 333, 111726. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Boer, D.G.; Fang, S.; Tang, Z.; Pescarmona, P.P. Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate. Catalysts 2021, 11, 1346. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Koelewijn, R.; El Hariri El Nokab, M.; van der Wel, P.C.; Sebakhy, K.O.; Pescarmona, P.P. Binder-free Zeolite Beta Beads with Hierarchical Porosity: Synthesis and Application as Heterogeneous Catalysts for Anisole Acylation. ChemCatChem 2022, 14, e202200518. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Perez-Pariente, J. Synthesis of titanoaluminosilicates isomorphous to zeolite Beta, active as oxidation catalysts. Zeolites 1993, 13, 82–87. [Google Scholar] [CrossRef]
- Van der Waal, J.C.; Lin, P.; Rigutto, M.S.; Van Bekkum, H. Synthesis of aluminium free titanium silicate with the BEA structure using a new and selective template and its use as a catalyst in epoxidations. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 1093–1100. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wang, J.; Fan, W. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Maldonado, M.; Oleksiak, M.D.; Chinta, S.; Rimer, J.D. Controlling crystal polymorphism in organic-free synthesis of Na-zeolites. J. Am. Chem. Soc. 2013, 135, 2641–2652. [Google Scholar] [CrossRef]
- Palčić, A.; Moldovan, S.; El Siblani, H.; Vicente, A.; Valtchev, V. Defect sites in zeolites: Origin and healing. Adv. Sci. 2022, 9, 2104414. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Dib, E.; Nesterenko, N.; Dath, J.P.; Gilson, J.P.; Mintova, S. Silanol defect engineering and healing in zeolites: Opportunities to fine-tune their properties and performances. Chem. Soc. Rev. 2021, 50, 11156–11179. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Centi, G.; Lanzafame, P.; Perathoner, S. Disruptive catalysis by zeolites. Catal. Sci. Technol. 2016, 6, 2485–2501. [Google Scholar] [CrossRef]
- Wenten, I.G.; Dharmawijaya, P.T.; Aryanti, P.T.P.; Mukti, R.R. LTA zeolite membranes: Current progress and challenges in pervaporation. RSC Adv. 2017, 7, 29520–29539. [Google Scholar] [CrossRef]
- Qin, Z.; Hafiz, L.; Shen, Y.; Van Daele, S.; Boullay, P.; Ruaux, V.; Gilson, J.-P.; Valtchev, V. Defect-engineered zeolite porosity and accessibility. J. Mater. Chem. A 2020, 8, 3621–3631. [Google Scholar] [CrossRef]
- Wu, Q.; Luan, H.; Xiao, F.S. Targeted synthesis of zeolites from calculated interaction between zeolite structure and organic template. Natl. Sci. Rev. 2022, 9, nwac023. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Jain, R.; Rimer, J.D. In situ imaging of two-dimensional surface growth reveals the prevalence and role of defects in zeolite crystallization. Proc. Natl. Acad. Sci. USA 2020, 117, 28632–28639. [Google Scholar] [CrossRef]
- Lupulescu, A.I.; Rimer, J.D. In situ imaging of silicalite-1 surface growth reveals the mechanism of crystallization. Science 2014, 344, 729–732. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Jain, R.; Mallette, A.J.; Rimer, J.D. Controlling nucleation pathways in zeolite crystallization: Seeding conceptual methodologies for advanced materials design. J. Am. Chem. Soc. 2021, 143, 21446–21460. [Google Scholar] [CrossRef]
- Pollens, N.; Doppelhammer, N.; Asselman, K.; Thijs, B.; Jakoby, B.; Reichel, E.; Taulelle, F.; Martens, J.; Breynaert, E.; Kirschhock, C.E.A. A zeolite crystallisation model confirmed by in situ observation. Faraday Discuss. 2022, 235, 162–182. [Google Scholar] [CrossRef]
- Li, T.; Krumeich, F.; van Bokhoven, J.A. Where Does the Zeolite ZSM-5 Nucleation and Growth Start? The Effect of Aluminum. Cryst. Growth Des. 2021, 19, 2548–2551. [Google Scholar] [CrossRef]
- Yoshioka, T.; Liu, Z.; Iyoki, K.; Chokkalingam, A.; Yonezawa, Y.; Hotta, Y.; Ohnishi, R.; Matsuo, T.; Yanaba, Y.; Ohara, K.; et al. Ultrafast and continuous-flow synthesis of AFX zeolite via interzeolite conversion of FAU zeolite. React. Chem. Eng. 2021, 6, 74–81. [Google Scholar] [CrossRef]
- Yaping, Y.E.; Xiaoqiang, Z.; Weilan, Q.; Mingwen, W. Synthesis of pure zeolites from supersaturated silicon and aluminum alkali extracts from fused coal fly ash. Fuel 2008, 87, 1880–1886. [Google Scholar] [CrossRef]
- De Yoreo, J. A perspective on multistep pathways of nucleation. In Crystallization via Nonclassical Pathways Volume 1: Nucleation, Assembly, Observation & Application; American Chemical Society: Washington, DC, USA, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Jones, A.G.; Graham, P. Population balance modeling of particle formation during the chemical synthesis of zeolite crystals: Assessment of hydrothermal precipitation kinetics. Zeolites 1996, 16, 164–172. [Google Scholar] [CrossRef]
- Jorge, M.; Auerbach, S.M.; Monson, P.A. Modelling the thermal stability of precursor nanoparticles in zeolite synthesis. Mol. Phys. 2006, 104, 3513–3522. [Google Scholar] [CrossRef]
- Asselman, K.; Pellens, N.; Thijs, B.; Doppelhammer, N.; Haouas, M.; Taulelle, F.; Martens, J.; Breyhaert, E.; Kirschhock, C.E. Ion-Pairs in Aluminosilicate-Alkali Synthesis Liquids Determine the Aluminum Content and Topology of Crystallizing Zeolites. Chem. Mater. 2022, 34, 7150–7158. [Google Scholar] [CrossRef]
- Kumar, A.; Molinero, V. Two-step to one-step nucleation of a zeolite through a metastable gyroid mesophase. J. Phys. Chem. Lett. 2018, 9, 5692–5697. [Google Scholar] [CrossRef]
- Thompson, R.W. Nucleation, growth, and seeding in zeolite synthesis. In Verified Syntheses of Zeolitic Materials; Elsevier: Amsterdam, The Netherlands, 2001; pp. 21–23. [Google Scholar] [CrossRef]
- Kumar, M.; Li, R.; Rimer, J.D. Assembly and evolution of amorphous precursors in zeolite L crystallization. Chem. Mater. 2016, 28, 1714–1727. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Ford, M.H.; Monson, P.A. New insights into zeolite formation from molecular modeling. Curr. Opin. Colloid Interface Sci. 2005, 10, 220–225. [Google Scholar] [CrossRef]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of zeolite growth: New findings and open question. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Kumar, M.; Choudhary, M.K.; Rimer, J.D. Transient modes of zeolite surface growth from 3D gel-like islands to 2D single layers. Nat. Commun. 2018, 9, 2129. [Google Scholar] [CrossRef]
- Yue, Q.; Kutukova, K.; Li, A.; Čejka, J.; Zschech, E.; Opanasenko, M. Controllable Zeolite AST Crystallization: Between Classical and Reversed Crystal Growth. Chem.-A Eur. J. 2022, 28, e202200590. [Google Scholar] [CrossRef]
- Yao, J.; Huang, Y.; Wang, H. Controlling zeolite structures and morphologies using polymer networks. J. Mater. Chem. 2010, 20, 9827–9831. [Google Scholar] [CrossRef]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W. Early stage reversed crystal growth of zeolite A and its phase transformation to sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef]
- Boer, D.G.; Asgar Pour, Z.; Langerak, J.; Bakker, B.; Pescarmona, P.P. Binderless Faujasite Beads with Hierarchical Porosity for Selective CO2 Adsorption for Biogas Upgrading. Molecules 2023, 28, 2198. [Google Scholar] [CrossRef]
- Zhou, W. Reversed crystal growth. Crystals 2018, 9, 7. [Google Scholar] [CrossRef]
- Zhang, X.L.; Qiu, L.F.; Ding, M.Z.; Hu, N.; Zhang, F.; Zhou, R.F.; Chen, X.S.; Kita, H. Preparation of zeolite T membranes by a two-step temperature process for CO2 separation. Ind. Eng. Chem. Res. 2013, 52, 16364–16374. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, D.; Jiang, G. Synthesis of zeolite NaA at room temperature: The effect of synthesis parameters on crystal size and its size distribution. Adv. Powder Technol. 2013, 24, 689–696. [Google Scholar] [CrossRef]
- Freeman, E.E.; Neeway, J.J.; Motkuri, R.K.; Rimer, J.D.; Mpourmpakis, G. Understanding initial zeolite oligomerization steps with first principles calculations. AIChE J. 2020, 66, e17107. [Google Scholar] [CrossRef]
- Houlleberghs, M.; Breynaert, E.; Asselman, K.; Vaneeckhaute, E.; Radhakrishnan, S.; Anderson, M.W.; Taulelle, F.; Haouas, M.; Martens, J.A.; Kirschhock, C.E. Evolution of the crystal growth mechanism of zeolite W (MER) with temperature. Microporous Mesoporous Mater. 2019, 274, 379–384. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, J.; Wang, Q. Controllable synthesis of hierarchical ZSM-5 for hydroconversion of vegetable oil to aviation fuel-like hydrocarbons. RSC Adv. 2017, 7, 46109–46117. [Google Scholar] [CrossRef]
- Xia, Q.-H.; Song, J.; Kawi, S.; Li, L. Characterization and morphological control of β zeolite synthesized in a fluoride medium. Stud. Surf. Sci. Catal. 2004, 154, 195–202. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasnida, N.; Nik, W.W. Preparation of Zeolite Y Using Local Raw Material Rice Husk as a Silica Source. J. Sci. Res. 2009, 1, 285–291. [Google Scholar] [CrossRef]
- Di Giuseppe, D. Characterization of fibrous mordenite: A first step for the evaluation of its potential toxicity. Crystals 2020, 10, 769. [Google Scholar] [CrossRef]
- Mao, Y.; Zhou, Y.; Wen, H.; Xie, J.; Zhang, W.; Wang, J. Morphology-controlled synthesis of large mordenite crystals. New J. Chem. 2014, 38, 3295–3301. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Sánchez, M.; Díaz, R.D.; Córdova, T.; González, G.; Ruette, F. Study of template interactions in MFI and MEL zeolites using quantum methods. Microporous Mesoporous Mater. 2015, 203, 91–99. [Google Scholar] [CrossRef]
- Cubillas, P.; Stevens, S.M.; Blake, N.; Umemura, A.; Chong, C.B.; Terasaki, O.; Anderson, M.W. AFM and HRSEM invesitigation of zeolite A crystal growth. Part 1: In the absence of organic additives. J. Phys. Chem. C 2011, 115, 12567–12574. [Google Scholar] [CrossRef]
- Corma, A.; Zones, S.; Cejka, J. Zeolites and Catalysis: Synthesis, Reactions and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Smith, R.L.; Sławiński, W.A.; Lind, A.; Wragg, D.S.; Cavka, J.H.; Arstad, B.; Fjellvåg, H.; Attfield, M.P.; Akporiaye, D.; Anderson, M.W. Nanoporous intergrowths: How crystal growth dictates phase composition and hierarchical structure in the CHA/AEI system. Chem. Mater. 2015, 27, 4205–4215. [Google Scholar] [CrossRef]
- John, N.S.; Stevens, S.M.; Terasaki, O.; Anderson, M.W. Evolution of surface morphology with introduction of stacking faults in zeolites. Chem.-A Eur. J. 2010, 16, 2220–2230. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Li, L.; Yang, J.; Fu, W.; Luo, Y.; Lu, J.; Zhang, Y.; Zhou, L. Highly selective zeolite T membranes with different ERI stacking faults for pervaporative dehydration of ethanol. J. Membr. Sci. 2021, 638, 119701. [Google Scholar] [CrossRef]
- Lupulescu, A.I.; Kumar, M.; Rimer, J.D. A facile strategy to design zeolite L crystals with tunable morphology and surface architecture. J. Am. Chem. Soc. 2013, 135, 6608–6617. [Google Scholar] [CrossRef]
- Hould, N.D.; Foster, A.; Lobo, R.F. Zeolite beta mechanisms of nucleation and growth. Microporous Mesoporous Mater. 2011, 142, 104–115. [Google Scholar] [CrossRef]
- Wu, R.; Fan, T.; Chen, J.; Li, Y. Synthetic factors affecting the scalable production of zeolitic imidazolate frameworks. ACS Sustain. Chem. Eng. 2019, 7, 3632–3646. [Google Scholar] [CrossRef]
- Nazari, M.; Yaripour, F.; Shifteh, S. Systematic evaluation and optimization of crystallization conditions for an ethanol-templated ZSM-5 zeolite using response surface methodology. Adv. Powder Technol. 2021, 32, 4621–4634. [Google Scholar] [CrossRef]
- Shamzhy, M.; Opanasenko, M.; Concepción, P.; Martínez, A. New trends in tailoring active sites in zeolite-based catalysts. Chem. Soc. Rev. 2019, 48, 1095–1149. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J. Recent Advances in the Synthesis and Application of Two-Dimensional Zeolites. Adv. Energy Mater. 2016, 6, 1600441. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Cejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Čejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef]
- Zhou, W. Microscopic study of crystal defects enriches our knowledge of materials chemistry. J. Mater. Chem. 2008, 18, 5321–5325. [Google Scholar] [CrossRef]
- Wright, P.A.; Zhou, W.; Pérez-Pariente, J.; Arranz, M. Direct observation of growth defects in zeolite beta. J. Am. Chem. Soc. 2005, 127, 494–495. [Google Scholar] [CrossRef]
- Karwacki, L.; Stavitski, E.; Kox, M.H.; Kornatowski, J.; Weckhuysen, B.M. Intergrowth structure of zeolite crystals as determined by optical and fluorescence microscopy of the template-removal process. Angew. Chem. 2007, 119, 7366–7369. [Google Scholar] [CrossRef]
- Silaghi, M.C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, H.T.; Jeong, H.K. Defect-dependent stability of highly propylene-selective zeolitic-imidazolate framework ZIF-8 membranes. J. Membr. Sci. 2017, 529, 105–113. [Google Scholar] [CrossRef]
- Han, R.; Sholl, D.S. Computational model and characterization of stacking faults in ZIF-8 polymorphs. J. Phys. Chem. C 2016, 120, 27380–27388. [Google Scholar] [CrossRef]
- Deng, A.; Shen, X.; Wan, Z.; Li, Y.; Pang, S.; He, X.; Caro, J.; Huang, A. Elimination of Grain Boundary Defects in Zeolitic Imidazolate Framework ZIF-95 Membrane via Solvent-Free Secondary Growth. Angew. Chem. 2021, 133, 25667–25671. [Google Scholar] [CrossRef]
- Resasco, D.E.; Crossley, S.P.; Wang, B.; White, J.L. Interaction of water with zeolites: A review. Catal. Rev. 2021, 63, 302–362. [Google Scholar] [CrossRef]
- Kumakiri, I.; Sasaki, Y.; Shimidzu, W.; Hashimoto, K.; Kita, H.; Yamaguchi, T.; Nakao, S.I. Micro-structure change of polycrystalline FAU zeolite membranes during a hydrothermal synthesis in a dilute solution. Microporous Mesoporous Mater. 2018, 272, 53–60. [Google Scholar] [CrossRef]
- Anderson, M.W.; Pachis, K.S.; Prébin, F.; Carr, S.W.; Terasaki, O.; Ohsuna, T.; Alfreddson, V. Intergrowths of cubic and hexagonal polytypes of faujasitic zeolites. Chem. Commun. 1991, 23, 1660–1664. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Sinclair, P.E.; Sokol, A.A. A model for the formation of point defects in zeolites. Radiat. Eff. Defects Solids 1999, 151, 235–241. [Google Scholar] [CrossRef]
- Li, J.; Mayoral, A.; Kubota, Y.; Inagaki, S.; Yu, J.; Terasaki, O. Direct TEM Observation of Vacancy-Mediated Heteroatom Incorporation into a Zeolite Framework: Towards Microscopic Design of Zeolite Catalysts. Angew. Chem. 2022, 134, e202211196. [Google Scholar] [CrossRef]
- Rimer, J.D.; Kumar, M.; Li, R.; Lupulescu, A.I.; Oleksiak, M.D. Tailoring the physicochemical properties of zeolite catalysts. Catal. Sci. Technol. 2014, 4, 3762–3771. [Google Scholar] [CrossRef]
- Dib, E.; Grand, J.; Gedeon, A.; Mintova, S.; Fernandez, C. Control the position of framework defects in zeolites by changing the symmetry of organic structure directing agents. Microporous Mesoporous Mater. 2021, 315, 110899. [Google Scholar] [CrossRef]
- Conroy, B.; Nayak, R.; Hidalgo, A.L.R.; Millar, G.J. Evaluation and application of machine learning principles to Zeolite LTA synthesis. Microporous Mesoporous Mater. 2022, 335, 111802. [Google Scholar] [CrossRef]
- Jensen, Z.; Kim, E.; Kwon, S.; Gani, T.Z.; Román-Leshkov, Y.; Moliner, M.; Corma, A.; Olivetti, E. A machine learning approach to zeolite synthesis enabled by automatic literature data extraction. ACS Cent. Sci. 2019, 5, 892–899. [Google Scholar] [CrossRef]
- Yang, S.; Lach-hab, M.; Vaisman, I.I.; Blaisten-Barojas, E. Identifying zeolite frameworks with a machine learning approach. J. Phys. Chem. C 2009, 113, 21721–21725. [Google Scholar] [CrossRef]
- Ma, S.; Shang, C.; Wang, C.M.; Liu, Z.P. Thermodynamic rules for zeolite formation from machine learning based global optimization. Chem. Sci. 2020, 11, 10113–10118. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P. Production of Highly Dispersed Ni within Nickel Silicate Materials with the MFI Structure for the Selective Hydrogenation of Olefins. Ind. Eng. Chem. Res 2019, 58, 8597–8611. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P.A. Dispersed Ni-Doped Aegirine Nanocatalysts for the Selective Hydrogenation of Olefinic Molecules. ACS Appl. Nano Mater 2018, 1, 6269–6280. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M. Formation and Structural Analysis of Ultra Low Density Silica Based Aerogels. Master’s Thesis, University of Siegen, Siegen, Germany, 2018. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Sebakhy, K.O. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials 2022, 11, 1494. [Google Scholar] [CrossRef] [PubMed]



| Zeolitic Framework | Example | Morphology | Synthesis Conditions | Ref. |
|---|---|---|---|---|
| MER | Zeolite W | Lamellar cubes | 90 °C | [42] |
| Elongated cubes | 175 °C | [42] | ||
| MFI | ZSM-5 | Coffin shape | Conventional | [5] |
| Microspheres | In the presence of carbon nanotubes | [43] | ||
| BEA | Zeolite Beta | Stacking structure | Conventional | [5] |
| Uniform prism-like crystals | F- medium | [44] | ||
| FAU | Zeolite Y | Octahedrons | Conventional | [5] |
| Microspheres | Seeding technique | [45] | ||
| MOR | Mordenite | Fiber | Conventional | [46] |
| bulky sphere, circular pie, flat prism, hexagonal star-like prism, and ellipsoid | acid-catalyzed hydrolysis and tetraethyl ammonium hydroxide as a template | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgar Pour, Z.; Alassmy, Y.A.; Sebakhy, K.O. A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps. Crystals 2023, 13, 959. https://doi.org/10.3390/cryst13060959
Asgar Pour Z, Alassmy YA, Sebakhy KO. A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps. Crystals. 2023; 13(6):959. https://doi.org/10.3390/cryst13060959
Chicago/Turabian StyleAsgar Pour, Zahra, Yasser A. Alassmy, and Khaled O. Sebakhy. 2023. "A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps" Crystals 13, no. 6: 959. https://doi.org/10.3390/cryst13060959
APA StyleAsgar Pour, Z., Alassmy, Y. A., & Sebakhy, K. O. (2023). A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps. Crystals, 13(6), 959. https://doi.org/10.3390/cryst13060959