Molecular Structures and Intermolecular Hydrogen Bonding of Silylated 2-Aminopyrimidines
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Syntheses and Characterization
3. Results and Discussion
3.1. Syntheses
3.2. Raman Spectroscopy
3.3. Molecular Structures
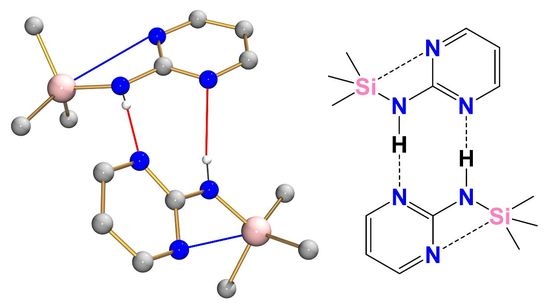
3.4. Intermolecular N–H···N Hydrogen Bonding
3.5. Reaction with CO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | 1 | 2 1 | (3)2 · (THF) 2 | 4 · (Anisole) 3 |
|---|---|---|---|---|
| Formula | C7H13N3Si | C10H14N6Si | C30H38N18OSi2 | C23H24N12OSi |
| Moiety formula | C7H13N3Si | C10H14N6Si | 2 (C13H15N9Si), C4H8O | C16H16N12Si, C7H8O |
| Mr | 167.29 | 246.36 | 722.96 | 512.63 |
| T (K) | 180(2) | 180(2) | 180(2) | 180(2) |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | monoclinic | triclinic | triclinic | monoclinic |
| Space group | P21/c | P | P | P21/c |
| a (Å) | 9.4337(3) | 8.6757(4) | 10.0640(3) | 15.9611(2) |
| b (Å) | 19.5412(6) | 8.8740(4) | 11.1921(3) | 21.1674(3) |
| c (Å) | 10.7349(3) | 9.0989(4) | 18.6757(5) | 22.6832(3) |
| α (°) | 90 | 64.211(3) | 72.898(2) | 90 |
| β (°) | 100.794(3) | 75.463(3) | 89.000(2) | 97.740(1) |
| γ (°) | 90 | 79.194(3) | 63.346(2) | 90 |
| V (Å3) | 1943.92(10) | 608.08(5) | 1780.70(9) | 7593.81(18) |
| Z | 8 | 2 | 2 | 12 |
| ρcalc (g·cm−1) | 1.14 | 1.35 | 1.35 | 1.35 |
| μMoKα (mm−1) | 0.2 | 0.2 | 0.2 | 0.1 |
| F (000) | 720 | 260 | 760 | 3216 |
| θmax (°), Rint | 28.0, 0.0377 | 28.0, 0.0232 | 28.0, 0.0319 | 27.0, 0.0428 |
| Completeness | 100% | 99.8% | 99.9% | 99.9% |
| Reflns collected | 17,865 | 9797 | 33,018 | 130,089 |
| Reflns unique | 4699 | 2937 | 8598 | 16,561 |
| Restraints | 0 | 0 | 24 | 0 |
| Parameters | 214 | 165 | 527 | 1150 |
| GoF | 1.097 | 1.080 | 1.063 | 1.108 |
| R1, wR2 [I > 2σ(I)] | 0.0367, 0.0876 | 0.0308, 0.0770 | 0.0385, 0.0959 | 0.0407, 0.0939 |
| R1, wR2 (all data) | 0.0495, 0.0957 | 0.0336, 0.0792 | 0.0462, 0.1019 | 0.0506, 0.1010 |
| Largest peak/hole (e·Å−3) | 0.24, −0.23 | 0.25, −0.25 | 0.28, −0.31 | 0.27, −0.35 |
References
- Kroke, E.; Li, Y.; Konetschny, C.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. Rep. 2000, 26, 97–199. [Google Scholar] [CrossRef]
- Völger, K.; Hauser, R.; Kroke, E.; Riedel, R.; Ikuhara, Y.; Iwamoto, Y. Synthesis and characterization of novel non-oxide sol-gel derived mesoporous amorphous Si-C-N membranes. J. Ceram. Soc. Jpn. 2006, 114, 567–570. [Google Scholar] [CrossRef]
- Krüger, C.; Rochow, E. Polyorganosilazanes. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 3179–3189. [Google Scholar] [CrossRef]
- Kraushaar, K.; Wiltzsch, C.; Wagler, J.; Böhme, U.; Schwarzer, A.; Roewer, G.; Kroke, E. From CO2 to Polysiloxanes: Di(carbamoyloxy)silanes Me2Si[(OCO)NRR’]2 as Precursors for PDMS. Organometallics 2012, 31, 4779–4785. [Google Scholar] [CrossRef]
- Kraushaar, K.; Schmidt, D.; Schwarzer, A.; Kroke, E. Chapter Four—Reactions of CO2 and CO2 Analogs (CXY with X, Y=O, S, NR) with Reagents Containing Si–H and Si–N Units. In CO2 Chemistry; Aresta, M., van Eldik, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 117–162. [Google Scholar] [CrossRef]
- Herbig, M.; Gevorgyan, L.; Pflug, M.; Wagler, J.; Schwarzer, S.; Kroke, E. CO2 Capture with Silylated Ethanolamines and Piperazines. ChemistryOpen 2019, 9, 894–902. [Google Scholar] [CrossRef]
- Wiltzsch, C.; Kraushaar, K.; Schwarzer, A.; Kroke, E. CO2 as an Oxygen Source for Polysiloxanes—Preparation, Crystal Structure and Thermal Decomposition of Two Novel Silylcarbamates. Z. Naturforsch. B 2011, 66, 917–922. [Google Scholar] [CrossRef]
- Gründler, F.; Scholz, H.; Herbig, M.; Schwarzer, S.; Wagler, J.; Kroke, E. Formation of Aromatic O-Silylcarbamates from Aminosilanes and Their Subsequent Thermal Decomposition with Formation of Isocyanates. Eur. J. Inorg. Chem. 2021, 2021, 2211–2224. [Google Scholar] [CrossRef]
- Kraushaar, K.; Herbig, M.; Schmidt, D.; Wagler, J.; Böhme, U.; Kroke, E. Insertion of phenyl isocyanate into mono- and diaminosilanes. Z. Naturforsch. B 2017, 72, 909–921. [Google Scholar] [CrossRef]
- Schöne, D.; Gerlach, D.; Wiltzsch, C.; Brendler, E.; Heine, T.; Kroke, E.; Wagler, J. A Distorted Trigonal Antiprismatic Cationic Silicon Complex with Ureato Ligands: Syntheses, Crystal Structures and Solid State 29Si NMR Properties. Eur. J. Inorg. Chem. 2010, 2010, 461–467. [Google Scholar] [CrossRef]
- Ryll, C.; Kraushaar, K.; Wagler, J.; Brendler, E.; Kroke, E. Disilanes with Pentacoordinate Si Atoms by Carbon Dioxide Insertion into Aminodisilanes: Syntheses, Molecular Structures, and Dynamic Behavior. ACS Omega 2022, 7, 9527–9536. [Google Scholar] [CrossRef]
- Herbig, M.; Böhme, U.; Kroke, E. Lactamomethylsilanes—Synthesis, Structures, and Reactivity towards CO2 and Phenylisocyanate. Z. Anorg. Allg. Chem. 2019, 645, 377–387. [Google Scholar] [CrossRef]
- Herbig, M.; Kroke, E. Synthesis and spectroscopic properties of iminosilanes. Chem. Data Collect. 2023, 44, 100992. [Google Scholar] [CrossRef]
- Herbig, M.; Böhme, U.; Schwarzer, A.; Kroke, E. Formation of 1-aza-2-silacyclopentanes and unexpected products from the insertion of phenylisocyanate into 2,2-dimethyl-1-(trimethylsilyl)-1-aza-2-sila-cyclo-pentane. Main Group Met. Chem. 2018, 41, 11–19. [Google Scholar] [CrossRef]
- Herbig, M.; Böhme, U.; Kroke, E. Insertion of CO2 and related heteroallenes into the Si-N-bond of methyl(N-morpholino)silanes. Inorg. Chim. Acta 2018, 473, 20–28. [Google Scholar] [CrossRef]
- Jin, G.; Jones, C.; Junk, P.; Lippert, K.; Roseab, R.; Stasch, A. Synthesis and characterisation of bulky guanidines and phosphaguanidines: Precursors for low oxidation state metallacycles. New J. Chem. 2009, 33, 64–75. [Google Scholar] [CrossRef]
- Lysenko, S.; Daniliuc, C.; Jones, P.; Tamm, M. Tungsten alkylidyne complexes with ancillary imidazolin-2-iminato and imidazolidin-2-iminato ligands and their use in catalytic alkyne metathesis. J. Organomet. Chem. 2013, 744, 7–14. [Google Scholar] [CrossRef]
- Deka, H.; Fridman, N.; Eisen, M. A Sacrificial Iminato Ligand in the Catalytic Cyanosilylation of Ketones Promoted by Organoactinide Complexes. Inorg. Chem. 2022, 61, 3598–3606. [Google Scholar] [CrossRef]
- Seidel, F.; Tomizawa, I.; Nozaki, K. Expedient Synthetic Identification of a P-Stereogenic Ligand Motif for the Palladium-Catalyzed Preparation of Isotactic Polar Polypropylenes. Angew. Chem. Int. Ed. 2020, 59, 22591–22601. [Google Scholar] [CrossRef]
- Oakley, S.; Coles, M.; Hitchcock, P. Poly(guanidyl)silanes as a new class of chelating, N-based ligand. Dalton Trans. 2004, 33, 1113–1114. [Google Scholar] [CrossRef]
- Mück, F.; Baus, J.; Ulmer, A.; Burschka, C.; Tacke, R. Reactivity of the Donor-Stabilized Guanidinatosilylene [ArNC(NMe2)NAr]Si[N(SiMe3)2] (Ar = 2,6-Diisopropylphenyl). Eur. J. Inorg. Chem. 2016, 2016, 1660–1670. [Google Scholar] [CrossRef]
- von Wolff, N.; Lefèvre, G.; Berthet, J.; Thuéry, P.; Cantat, T. Implications of CO2 Activation by Frustrated Lewis Pairs in the Catalytic Hydroboration of CO2: A View Using N/Si+ Frustrated Lewis Pairs. ACS Catal. 2016, 6, 4526–4535. [Google Scholar] [CrossRef]
- Reiter, D.; Frisch, P.; Wendel, D.; Hörmann, F.; Inoue, S. Oxidation reactions of a versatile, two-coordinate, acyclic iminosiloxysilylene. Dalton Trans. 2020, 49, 7060–7068. [Google Scholar] [CrossRef]
- Inoue, S.; Leszczyn’ska, K. An Acyclic Imino-Substituted Silylene: Synthesis, Isolation, and its Facile Conversion into a Zwitterionic Silaimine. Angew. Chem. Int. Ed. 2012, 51, 8589–8593. [Google Scholar] [CrossRef]
- Coles, M.; Sözerli, S.; Smith, J.; Hitchcock, P.; Day, I. An Ether-Free, Internally Coordinated Dialkylcalcium(II) Complex. Organometallics 2009, 28, 1579–1581. [Google Scholar] [CrossRef]
- Smart, K.; Grellier, M.; Coppel, Y.; Vendier, L.; Mason, S.; Capelli, S.; Albinati, A.; Montiel-Palma, V.; Muñoz-Hernández, M.; Sabo-Etienne, S. Nature of Si−H Interactions in a Series of Ruthenium Silazane Complexes Using Multinuclear Solid-State NMR and Neutron Diffraction. Inorg. Chem. 2014, 53, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Strunden, T.; Greb, L. Silicon Carbamates by CO2 Fixation: Brønsted Acid Labile Precursor of a Lewis Superacid. Inorg. Chem. 2022, 61, 15693–15698. [Google Scholar] [CrossRef]
- Kociok-Köhn, G.; Molloy, K.; Price, G.; Smith, D. Structural characterisation of trimethylsilyl-protected DNA bases. Supramol. Chem. 2008, 20, 697–707. [Google Scholar] [CrossRef]
- Bertolasi, V.; Boaretto, R.; Chierotti, M.; Gobettoc, R.; Sostero, S. Synthesis, characterization and reactivity of new complexes of titanium and zirconium containing a potential tridentate amidinato-cyclopentadienyl ligand. Dalton Trans. 2007, 36, 5179–5189. [Google Scholar] [CrossRef]
- Oberthür, M.; Arndt, P.; Kempe, R. Synthesis and Structure of Mononuclear Titanium Complexes Containing ansa-Aminopyridinato Ligands. Chem. Ber. 1996, 129, 1087–1091. [Google Scholar] [CrossRef]
- Herzog, S.; Dehnert, J. Eine rationelle anaerobe Arbeitsmethode. Z. Chem. 1964, 4, 1–11. [Google Scholar] [CrossRef]
- Böhme, U. Inertgastechnik; De Gruyter: Oldenbourg, Germany, 2020. [Google Scholar] [CrossRef]
- Herbig, M.; Kroke, E. Low Cost Apparatus for Rapid Boiling Point Determination of Small Air Sensitive Samples under Inert Atmosphere. Thermochim. Acta 2017, 654, 81–84. [Google Scholar] [CrossRef]
- Cie, S. X-RED and X-AREA; Stoe & Cie: Darmstadt, Germany, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Cason, C.; Fröhlich, T.; Lipka, C. POV-RAY (Version 3.7); Persistence of Vision Raytracer Pty. Ltd.: Williamstown, Australia, 1994–2004. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Kaftory, M.; Kapon, M.; Botoshansky, M. The Structural Chemistry of Organosilicon Compounds. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; Wiley & Sons: Chichester, UK, 1998; pp. 181–265. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Merzweiler, K.; Lechner, B.-D. (Martin-Luther-Universität Halle-Wittenberg, Halle, Germany). Private communication, 2022. Private Communication to the Cambridge Structure Database. deposition number CCDC 2182492, CSD refcode CEKWIC. [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Wagner, C.; Merzweiler, K. Novel Dinuclear Tin(II) and Lead(II) Compounds with 2-Pyridyl Functionalized Silylamido Ligands. Z. Anorg. Allg. Chem. 2017, 643, 938–944. [Google Scholar] [CrossRef]
- Pérez, E.; Santos, R.; Gambardella, M.; de Macedo, L.; Rodrigues-Filho, U.; Launay, J.; Franco, D. Activation of Carbon Dioxide by Bicyclic Amidines. J. Org. Chem. 2004, 69, 8005–8011. [Google Scholar] [CrossRef]
- Breederveld, H. The reaction of dialkylaminosilanes with carbon dioxide and with carbon disulphide. Recl. Trav. Chim. Pays-Bas 1962, 81, 276–278. [Google Scholar] [CrossRef]
- Cragg, R.; Lappert, M. Amino-derivatives of metals and metalloids. Part IV. Aminosilylation and aminophosphination of some unsaturated substrates. J. Chem. Soc. A 1966, 82–85. [Google Scholar] [CrossRef]
- Ehrlich, L.; Gericke, R.; Brendler, E.; Wagler, J. (2-Pyridyloxy)silanes as Ligands in Transition Metal Coordination Chemistry. Inorganics 2018, 6, 119. [Google Scholar] [CrossRef]
- Kuß, S.; Brendler, E.; Wagler, J. Molecular Structures of the Pyridine-2-olates PhE(pyO)3 (E = Si, Ge, Sn)—[4+3]-Coordination at Si, Ge vs. Heptacoordination at Sn. Crystals 2022, 12, 1802. [Google Scholar] [CrossRef]
- Seidel, A.; Weigel, M.; Ehrlich, L.; Gericke, R.; Brendler, E.; Wagler, J. Molecular Structures of the Silicon Pyridine-2-(thi)olates Me3Si(pyX), Me2Si(pyX)2 and Ph2Si(pyX)2 (py = 2-Pyridyl, X = O, S), and Their Intra- and Intermolecular Ligand Exchange in Solution. Crystals 2022, 12, 1054. [Google Scholar] [CrossRef]
- Seidel, A.; Gericke, R.; Brendler, E.; Wagler, J. Copper Complexes of Silicon Pyridine-2-olates RSi(pyO)3 (R = Me, Ph, Bn, Allyl) and Ph2Si(pyO)2. Inorganics 2023, 11, 2. [Google Scholar] [CrossRef]
- Baus, J.; Burschka, C.; Bertermann, R.; Guerra, C.; Bickelhaupt, F.; Tacke, R. Neutral Six-Coordinate and Cationic Five-Coordinate Silicon(IV) Complexes with Two Bidentate Monoanionic N,S-Pyridine-2-thiolato(-) Ligands. Inorg. Chem. 2013, 52, 10664–10676. [Google Scholar] [CrossRef] [PubMed]
- Wächtler, E.; Gericke, R.; Kutter, S.; Brendler, E.; Wagler, J. Molecular structures of pyridinethiolato complexes of Sn(II), Sn(IV), Ge(IV), and Si(IV). Main Group Met. Chem. 2013, 36, 181–191. [Google Scholar] [CrossRef]
- Khodov, I.; Sobornova, V.; Mulloyarova, V.; Belov, K.; Dyshin, A.; de Carvalho, L.B.; Tolstoy, P.; Kiselev, M. Exploring the Conformational Equilibrium of Mefenamic Acid Released from Silica Aerogels via NMR Analysis. Int. J. Mol. Sci. 2023, 24, 6882. [Google Scholar] [CrossRef]
- Rovai, R.; Lehmann, C.W.; Bradley, J.S. Non-Oxide Sol–Gel Chemistry: Preparation from Tris(dialkylamino)silazanes of a Carbon-Free, Porous, Silicon Diimide Gel. Angew. Chem. Int. Ed. 1999, 38, 2036–2038. [Google Scholar] [CrossRef]
- Lippe, K.; Wagler, J.; Kroke, E.; Herkenhoff, S.; Ischenko, V.; Woltersdorf, J. Cyclic Silylcarbodiimides as Precursors for Porous Si/C/N Materials: Formation, Structures, and Stabilities. Chem. Mater. 2009, 21, 3941–3949. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Kroke, E.; Herkenhoff, S. In situ synthesis of Si2N2O/Si3N4 composite ceramics using polysilyloxycarbodiimide precursors. J. Eur. Ceram. Soc. 2013, 33, 2181–2189. [Google Scholar] [CrossRef]
- Hossain, A.; Dey, A.; Seth, S.K.; Ray, P.P.; Ballester, P.; Pritchard, R.G.; Ortega-Castro, J.; Frontera, A.; Mukhopadhyay, S. Enhanced Photosensitive Schottky Diode Behavior of Pyrazine over 2-Aminopyrimidine Ligand in Copper(II)-Phthalate MOFs: Experimental and Theoretical Rationalization. ACS Omega 2018, 3, 9160–9171. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, N.; Huang, R.-B.; Zheng, L.-S. Series of Ag(I) Coordination Complexes Derived from Aminopyrimidyl Ligands and Dicarboxylates: Syntheses, Crystal Structures, and Properties. Cryst. Growth Des. 2010, 10, 3699–3709. [Google Scholar] [CrossRef]













| Vibration | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1590-1520 (aromatic CC, CN str.) | 1584 | 1580 | 1580 | 1580 |
| 1005-960 (aromatic CC, CN str.) | 992 | 986 | 986 | 986 |
| 650-630 | 641 | 651 | 651 | 651 |
| Compound | Atoms | Bond Length [Å] | Atoms | Bond Angle [°] |
|---|---|---|---|---|
| 1 | Si1–N1 | 1.7490(13) | N1-Si1-C5 | 112.66(7) |
| Si1–C5 | 1.8501(17) | N1-Si1-C6 | 110.28(7) | |
| Si1–C6 | 1.8499(18) | N1-Si1-C7 | 102.94(8) | |
| Si1–C7 | 1.8565(19) | C5-Si1-C6 | 109.77(10) | |
| C6-Si1-C7 | 111.10(10) | |||
| C5-Si1-C7 | 109.94(9) | |||
| Si2–N4 | 1.7485(13) | N4-Si2-C12 | 104.00(8) | |
| Si2–C12 | 1.8562(18) | N4-Si2-C13 | 111.12(7) | |
| Si2–C13 | 1.8544(16) | N4-Si2-C14 | 111.27(8) | |
| Si2–C14 | 1.8556(19) | C12-Si2-C13 | 108.99(9) | |
| C13-Si2-C14 | 111.19(8) | |||
| C12-Si2-C14 | 110.01(10) | |||
| 2 | Si1–N1 | 1.7377(10) | N1-Si1-N4 | 111.66(5) |
| Si1–N4 | 1.7439(10) | N1-Si1-C9 | 105.37(5) | |
| Si1–C9 | 1.8521(13) | N1-Si1-C10 | 112.33(5) | |
| Si1–C10 | 1.8481(12) | N4-Si1-C9 | 111.76(6) | |
| N4-Si1-C10 | 104.54(5) | |||
| C9-Si1-C10 | 111.35(6) | |||
| 3 | Si1–N1 | 1.7369(12) | N1-Si1-N4 | 104.81(6) |
| Si1–N4 | 1.7369(12) | N1-Si1-N7 | 105.72(6) | |
| Si1–N7 | 1.7338(11) | N4-Si1-N7 | 104.73(5) | |
| Si1–C13 | 1.8451(14) | N1-Si1-C13 | 112.00(6) | |
| N4-Si1-C13 | 115.55(6) | |||
| N7-Si1-C13 | 113.14(6) | |||
| Si2–N10 | 1.7332(11) | N10-Si2-N13 | 105.81(6) | |
| Si2–N13 | 1.7336(12) | N10-Si2-N16 | 104.46(5) | |
| Si2–N16 | 1.7438(12) | N13-Si2-N16 | 104.11(6) | |
| Si2–C26 | 1.8400(14) | N10-Si2-C26 | 113.22(6) | |
| N13-Si2-C26 | 112.49(6) | |||
| N16-Si2-C26 | 115.77(6) | |||
| 4 | Si1–N1 | 1.7218(13) | N1-Si1-N4 | 107.83(6) |
| Si1–N4 | 1.7180(12) | N1-Si1-N7 | 112.77(7) | |
| Si1–N7 | 1.7153(14) | N1-Si1-N10 | 107.28(6) | |
| Si1–N10 | 1.7189(13) | N4-Si1-N7 | 107.43(6) | |
| N4-Si1-N10 | 113.07(6) | |||
| N7-Si1-N10 | 108.57(6) | |||
| Si2–N13 | 1.7192(13) | N13-Si2-N16 | 109.10(6) | |
| Si2–N16 | 1.7181(12) | N13-Si2-N19 | 108.96(6) | |
| Si2–N19 | 1.7272(13) | N13-Si2-N22 | 108.53(6) | |
| Si2–N22 | 1.7196(12) | N16-Si2-N19 | 109.11(6) | |
| N16-Si2-N22 | 112.34(6) | |||
| N19-Si2-N22 | 108.75(6) | |||
| Si3–N25 | 1.7166(13) | N25-Si3-N28 | 107.78(6) | |
| Si3–N28 | 1.7158(13) | N25-Si3-N31 | 111.48(6) | |
| Si3–N31 | 1.7202(13) | N25-Si3-N34 | 107.66(6) | |
| Si3–N34 | 1.7200(13) | N28-Si3-N31 | 108.10(6) | |
| N28-Si3-N34 | 113.35(6) | |||
| N31-Si3-N34 | 108.52(6) |
| Compound | Atoms | Distance [Å] | Atoms | Distance [Å] |
|---|---|---|---|---|
| 1 | Si1···N2 | 3.0464(13) | Si2···N6 | 3.0035(12) |
| 2 | Si1···N2 | 3.0609(11) | Si1···N5 | 3.0450(11) |
| 3 | Si1···N3 | 2.9826(13) | Si2···N12 | 2.9990(12) |
| Si1···N6 | 3.1337(13) | Si2···N15 | 2.9773(18) | |
| Si1···N9 | 2.9452(12) | Si2···N18 | 3.1320(13) | |
| 4 | Si1···N2 | 3.0442(13) | Si2···N20 | 3.0120(13) |
| Si1···N5 | 3.0098(13) | Si2···N23 | 3.0494(14) | |
| Si1···N8 | 3.0792(13) | Si3···N26 | 3.0269(13) | |
| Si1···N11 | 3.0165(14) | Si3···N29 | 3.0718(15) | |
| Si2···N14 | 3.0453(13) | Si3···N32 | 3.0610(13) | |
| Si2···N17 | 2.9621(13) | Si3···N35 | 3.0307(14) |
| Compound | Atoms | Distance [Å] | Atoms | Angle [°] |
|---|---|---|---|---|
| 1 | N1–N5 | 3.1279(17) | N1-N5-C11 | 156.45(6) |
| N3–N4 | 3.0702(17) | C2-N3-N4 | 151.03(7) | |
| 2 | N1–N5 * | 3.2573(14) | ||
| N4–N6 ** | 3.3115(14) | N4-N6**-C6 ** | 157.88(6) | |
| 3 | N1–N2 * | 3.0607(17) | N1-N2 *-C4 * | 172.20(6) |
| N4–N14 | 3.0292(17) | N4-N14-C21 | 161.63(8) | |
| N5–N13 | 3.1112(16) | N13-N5-C8 | 170.77(6) | |
| N7–N17 ** | 3.1330(16) | N7-N17 **-C25 ** | 172.30(6) | |
| N8–N16 ** | 3.1037(16) | N16 **-N8-C12 | 172.39(6) | |
| N10–N11 *** | 3.0532(16) | N10-N11 ***-C17 *** | 177.81(6) | |
| 4 | N1–N18 | 3.0625(19) | ||
| N8–N16 | 3.1051(18) | |||
| N4–N32 * | 3.0728(17) | |||
| N6–N25 * | 3.0227(18) | |||
| N7–N36 * | 2.9871(19) | |||
| N2–N34 * | 3.0881(18) | |||
| N10–N14 | 3.0374(17) | |||
| N12–N19 | 3.1256(18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbig, M.; Kroke, E.; Wagler, J. Molecular Structures and Intermolecular Hydrogen Bonding of Silylated 2-Aminopyrimidines. Crystals 2023, 13, 990. https://doi.org/10.3390/cryst13070990
Herbig M, Kroke E, Wagler J. Molecular Structures and Intermolecular Hydrogen Bonding of Silylated 2-Aminopyrimidines. Crystals. 2023; 13(7):990. https://doi.org/10.3390/cryst13070990
Chicago/Turabian StyleHerbig, Marcus, Edwin Kroke, and Jörg Wagler. 2023. "Molecular Structures and Intermolecular Hydrogen Bonding of Silylated 2-Aminopyrimidines" Crystals 13, no. 7: 990. https://doi.org/10.3390/cryst13070990
APA StyleHerbig, M., Kroke, E., & Wagler, J. (2023). Molecular Structures and Intermolecular Hydrogen Bonding of Silylated 2-Aminopyrimidines. Crystals, 13(7), 990. https://doi.org/10.3390/cryst13070990