Water-Soluble CdTe/CdS Core/Shell Semiconductor Nanocrystals: How Their Optical Properties Depend on the Synthesis Methods
Abstract
:1. Introduction
2. Results
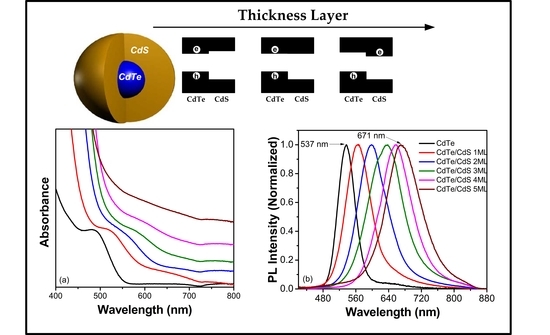
2.1. CdTe/CdS QDs Synthesized via the SILAR Method
2.2. CdTe/CdS QDs Synthesis via the One-Pot Approach
3. Discussions
4. Materials and Methods
4.1. Reagents
4.2. CdTe Synthesis
4.3. CdTe/CdS Synthesis Using the SILAR Method
4.4. CdTe/CdS Synthesis by the One-Pot Approach
4.5. Characterization
5. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hetsch, F.; Xu, X.; Wang, H.; Kershaw, S.V.; Rogach, A.L. Semiconductor nanocrystal quantum dots as solar cell components and photosensitizers: Material, charge transfer, and separation aspects of some device topologies. J. Phys. Chem. Lett. 2011, 2, 1879–1887. [Google Scholar] [CrossRef]
- Kamat, P.V. Quantum dot solar cells. The next big thing in photovoltaics. J. Phys. Chem. Lett. 2013, 4, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Ruhle, S.; Shalom, M.; Zaban, A. Quantum-dot-sensitized solar cells. ChemPhysChem 2010, 11, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Sargent, E.H. Colloidal Quantum Dots Optoelectronics and Photovoltaics; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Ann. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.; Johny, S.; Thomas, D.; Setua, S.; Menon, D.; Nair, S. Bio-conjugated luminescent quantum dots of doped ZnS: A cyto-friendly system for targeted cancer imaging. Nanotechnology 2009, 20, 065102. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.O.; Viol, L.C.d.S.; Ferreira, D.L.; Alves, J.L.A.; Schiavon, M.A. Estado da arte da síntese de semicondutores. Quím. Nova 2010, 33, 1933–1939. [Google Scholar] [CrossRef]
- Fontes, A.; Santos, B.S. Quantum Dots: Applications in Biology, 2nd ed.; Humana Press: New York, NY, USA, 2014; Volume 1, p. XI 258. [Google Scholar]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, Z.; Wang, Y.; Zhang, K.; Ji, X.; Lü, C.; Yang, B.; Gao, M. From water-soluble CdTe nanocrystals to fluorescent nanocrystal-polymer transparent composites using polymerizable surfactants. Adv. Mater. 2003, 15, 777–780. [Google Scholar] [CrossRef]
- Talapin, D.V.; Poznyak, S.K.; Gaponik, N.P.; Rogach, A.L.; Eychmüller, A. Synthesis of surface-modified colloidal semiconductor nanocrystals and study of photoinduced charge separation and transport in nanocrystal-polymer composites. Physica E 2002, 14, 237–241. [Google Scholar] [CrossRef]
- Rogach, A.L. Semiconductor Nanocrystal Quantum Dots; Rogach, A.L., Ed.; Springer: Wien, Austria, 2008. [Google Scholar]
- Aldeek, F.; Balan, L.; Medjahdi, G.; Roques-Carnes, T.; Malval, J.-P.; Mustin, C.; Ghanbaja, J.; Schneider, R. Enhanced optical properties of core/shell/shell CdTe/CdS/ZnO quantum dots prepared in aqueous solution. J. Phys. Chem. C 2009, 113, 19458–19467. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Preparation of water-soluble CdTe/CdS core/shell quantum dots with enhanced photostability. J. Lumin. 2007, 127, 721–726. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Piryatinski, A.; Nanda, J.; Treitak, S.; Zavadil, K.R.; Wallace, W.O.; Werder, D.; Klimov, V.I. Type-II core/shell CdS/Zn Senanocrystals: Synthesis, electronic structures, and spectroscopic properties. J. Am. Chem. Soc. 2007, 129, 11708–11719. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fisher, B.; Eisler, H.-J.; Bawendi, M. Type-II quantum dots: CdTe/CdSe (core/shell) and CdSe/ZnTe (core/shell) heterostructures. J. Am. Chem. Soc. 2003, 125, 11466–11467. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mohs, A.M.; Nie, S. Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat. Nanotechnol. 2008, 4, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Yu, J.-S. Selective synthesis of CdTe and high luminescence CdTe/CdS quantum dots: The effect of ligands. J. Colloid Interfaces Sci. 2009, 333, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Nanda, J.; Piryatinski, A.; Achermann, M.; Balet, L.P.; Bezel, I.V.; Anikeeva, P.O.; Tretiak, S.; Klimov, V.I. Light amplification using inverted core/shell nanocrystals: Towards lasing in the single-exciton regime. J. Phys. Chem. B 2004, 108, 10625–10630. [Google Scholar] [CrossRef]
- Shi, A.; Sun, J.; Zeng, Q.; Shao, C.; Sun, Z.; Li, H.; Kong, X.; Zhao, J. Photoluminescence quenching of CdTe/CdS core-shell quantum dots in aqueous solution by ZnO nanocrystals. J. Lumin. 2011, 131, 1536–1540. [Google Scholar] [CrossRef]
- Kuo, K.-T.; Liu, D.-M.; Chen, S.-Y.; Lin, C.-C. Core-shell CuInS2/ZnSquantum dots assembled on short ZnOnanowires with enhanced photo-conversion efficiency. J. Mater. Chem. 2009, 19, 6780–6788. [Google Scholar] [CrossRef]
- Kim, M.R.; Ma, D. Quantum-dot-based solar cells: Recent advances, strategies, and challenges. J. Phys. Chem. Lett. 2015, 6, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zou, L.; Fang, Z.; Zhu, W.; Zhong, X. One-pot synthesis of highly luminescent CdTe/CdS core/shell nanocrystals in aqueous phase. Nanotechnology 2008, 19, 135604–135611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Kong, X.; Sun, Y.; Zhang, Y.; Tu, L.; Zhao, J.; Zhang, H. Synthesis and optical properties of type II CdTe/CdS core/shell quantum dots in aqueous solution via successive ion layer adsorption and reaction. J. Phys. Chem. C 2008, 112, 8587–8593. [Google Scholar] [CrossRef]
- Van Embden, J.; Jasieniak, J.; Gómes, D.E.; Mulvaney, P.; Giersig, M. Review of the synthetic chemistry involved in the production of core/shell semiconductor nanocrystals. Aust. J. Chem. 2007, 60, 457–471. [Google Scholar] [CrossRef]
- Silva, F.O.; Carvalho, M.S.; Mendonça, R.; Macedo, W.A.A.; Balzuweit, K.; Reiss, P.; Schiavon, M.A. Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res. Lett. 2012, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Dorfs, D.; Franzl, T.; Osovsky, R.; Brumer, M.; Lifshitz, E.; Klar, T.A.; Eychmüller, A. Type I and type II nanoscale heterostructures based on CdTe nanocrystals: A comparative study. Small 2008, 4, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.-Y.; Gao, B.-R.; Pan, L.-Y.; Jiang, Y.; Chen, Q.-D.; Han, W.; Sun, H.-B. Transient absorption spectroscopic study on band-structure-type change in CdTe/CdS core-shell quantum dots. IEEE J. Quantum Electron. 2011, 47, 1177–1184. [Google Scholar] [CrossRef]
- Rogach, A.L. Nanocrystalline CdTe and CdTe(s) particles: Wet chemical preparation, size-dependent optical properties and perspective of optoelectronic applications. Mater. Sci. Eng. B 2000, 69, 435–440. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yield using a computer-controlled luminescence. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Cheng, C.-T.; Yu, J.-K.; Pu, S.-C.; Cheng, Y.-M.; Chou, P.-T. Spectroscopy and femtosecond dynamics of type-IICdSe/ZnTe core-shell semiconductor synthesized via the CdO precursor. J. Phys. Chem. B 2004, 108, 10687–10691. [Google Scholar] [CrossRef]
- Gui, R.; An, X.; Su, H.; Shen, W.; Chen, Z.; Wang, X. A near-infrared-emitting CdTe/CdS core/shell quantum dots-based off-on fluorescence sensor for highly selective and sensitive detection of Cd2+. Talanta 2012, 94, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Vale, B.R.C.; Vieira, K.O.; Sousa, J.C.L.; Ferrari, J.L.; Schiavon, M.A. Efeito da estrutura molecular de ligantes de superfícies em pontos quânticos de CdTe dispersos em água. Quím. Nova 2015, 38, 22–29. [Google Scholar]
- Resch-Genger, U.; Rurack, K. Determination of the photoluminescence quantum yield of dilute dye solutions (IUPAC technical report). Pure Appl. Chem. 2013, 85, 2005–2026. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]











© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, B.R.C.; Silva, F.O.; Carvalho, M.S.; Raphael, E.; Ferrari, J.L.; Schiavon, M.A. Water-Soluble CdTe/CdS Core/Shell Semiconductor Nanocrystals: How Their Optical Properties Depend on the Synthesis Methods. Crystals 2016, 6, 133. https://doi.org/10.3390/cryst6100133
Vale BRC, Silva FO, Carvalho MS, Raphael E, Ferrari JL, Schiavon MA. Water-Soluble CdTe/CdS Core/Shell Semiconductor Nanocrystals: How Their Optical Properties Depend on the Synthesis Methods. Crystals. 2016; 6(10):133. https://doi.org/10.3390/cryst6100133
Chicago/Turabian StyleVale, Brener R. C., Fernanda O. Silva, Melissa S. Carvalho, Ellen Raphael, Jefferson L. Ferrari, and Marco A. Schiavon. 2016. "Water-Soluble CdTe/CdS Core/Shell Semiconductor Nanocrystals: How Their Optical Properties Depend on the Synthesis Methods" Crystals 6, no. 10: 133. https://doi.org/10.3390/cryst6100133
APA StyleVale, B. R. C., Silva, F. O., Carvalho, M. S., Raphael, E., Ferrari, J. L., & Schiavon, M. A. (2016). Water-Soluble CdTe/CdS Core/Shell Semiconductor Nanocrystals: How Their Optical Properties Depend on the Synthesis Methods. Crystals, 6(10), 133. https://doi.org/10.3390/cryst6100133