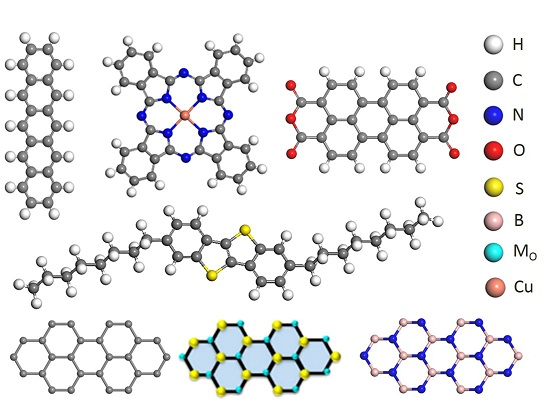
Van Der Waals Heterostructures between Small Organic Molecules and Layered Substrates
Abstract
:1. Introduction
2. Tuning the Orientation of Pentacene
3. Pentacene on Layered Substrates
4. CuPc on Layered Substrates
5. C8-BTBT on Layered Substrates
6. PTCDA on Layered Substrates
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sokolov, A.N.; Tee, B.C.; Bettinger, C.J.; Tok, J.B.H.; Bao, Z. Chemical and engineering approaches to enable organic field-effect transistors for electronic skin applications. Acc. Chem. Res. 2011, 45, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. Surface analytical studies of interfaces in organic semiconductor devices. Mater. Sci. Eng. R 2010, 68, 39–87. [Google Scholar] [CrossRef]
- Qian, C.; Sun, J.; Zhang, L.; Xie, H.; Huang, H.; Yang, J.; Gao, Y. Air-stable and high-performance organic field-effect transistors based on ordered, large-domain phthalocyanine copper thin film. Synth. Met. 2015, 210, 336–341. [Google Scholar] [CrossRef]
- Huang, W.; Yang, B.; Sun, J.; Liu, B.; Yang, J.; Zou, Y.; Gao, Y. Organic field-effect transistor and its photoresponse using a benzo [1,2-b:4,5-b′] difuran-based donor–acceptor conjugated polymer. Org. Electron. 2014, 15, 1050–1055. [Google Scholar] [CrossRef]
- Koch, N.; Vollmer, A.; Salzmann, I.; Nickel, B.; Weiss, H.; Rabe, J.P. Evidence for temperature-dependent electron band dispersion in pentacene. Phys. Rev. Lett. 2006, 96, 156803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, J.; Gao, S.; Hu, F.; He, D.; Wu, B.; Ji, W. Probing Carrier Transport and Structure-Property Relationship of Highly Ordered Organic Semiconductors at the Two-Dimensional Limit. Phys. Rev. Lett. 2016, 116, 016602. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, J.T.; Nagao, T.; Yaginuma, S.; Fujikawa, Y.; Al-Mahboob, A.; Nakajima, K.; Tromp, R.M. Thin bismuth film as a template for pentacene growth. Appl. Phys. Lett. 2005, 86, 073109. [Google Scholar] [CrossRef]
- Kakuta, H.; Hirahara, T.; Matsuda, I.; Nagao, T.; Hasegawa, S.; Ueno, N.; Sakamoto, K. Electronic structures of the highest occupied molecular orbital bands of a pentacene ultrathin film. Phys. Rev. Lett. 2007, 98, 247601. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, Y.; Pflaum, J.; Wee, A.T.S.; Chen, W. Nanoscale phase separation of a binary molecular system of copper phthalocyanine and di-indenoperylene on Ag (111). Appl. Phys. Lett. 2009, 95, 263309. [Google Scholar] [CrossRef]
- Zheng, F.; Park, B.N.; Seo, S.; Evans, P.G.; Himpsel, F.J. Orientation of pentacene molecules on SiO2: From a monolayer to the bulk. J. Chem. Phys. 2007, 126, 154702. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Choudhary, D.; Nickel, B.; Toccoli, T.; Chang, K.C.; Mayer, A.C.; Malliaras, G.G. Pentacene thin film growth. Chem. Mater. 2004, 16, 4497–4508. [Google Scholar] [CrossRef]
- Wang, C.; Irfan, I.; Turinske, A.J.; Gao, Y. Pinning of fullerene lowest unoccupied molecular orbital edge at the interface with standing up copper phthalocyanine. Thin Solid Films 2012, 525, 64–67. [Google Scholar] [CrossRef]
- He, T.; Ding, H.; Peor, N.; Lu, M.; Corley, D.A.; Chen, B.; Tour, J.M. Silicon/molecule interfacial electronic modifications. J. Am. Chem. Soc. 2008, 130, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.; Sim, S.H.; Lim, S.; Kim, K.S.; Hong, B.H.; Cho, K. Surface-directed molecular assembly of pentacene on monolayer graphene for high-performance organic transistors. J. Am. Chem. Soc. 2011, 133, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Li, H.; Huang, H.; Wong, S.L.; Lv, L.; Gao, Y.; Wee, A.T.S. Incorporating isolated molybdenum (Mo) atoms into bilayer epitaxial graphene on 4H-SiC (0001). ACS Nano 2013, 8, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wong, S.L.; Wang, Y.; Sun, J.T.; Gao, X.; Wee, A.T.S. Scanning tunneling microscope and photoemission spectroscopy investigations of bismuth on epitaxial graphene on SiC (0001). J. Phys. Chem. C 2014, 118, 24995–24999. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Wang, C.; Xu, X.; Li, Y.; Xie, F.; Gao, Y. Molecular orientation of copper phthalocyanine thin films on different monolayers of fullerene on SiO2 or highly oriented pyrolytic graphite. Appl. Phys. Lett. 2015, 106, 121603. [Google Scholar] [CrossRef]
- Wan, X.; Long, G.; Huang, L.; Chen, Y. Graphene–A promising material for organic photovoltaic cells. Adv. Mater. 2011, 23, 5342–5358. [Google Scholar] [CrossRef] [PubMed]
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Ye, P.D. Channel length scaling of MoS2 MOSFETs. ACS Nano 2012, 6, 8563–8569. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Konar, A.; Hwang, W.S.; Lee, J.H.; Lee, J.; Yang, J.; Jin, Y.W. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 2012, 3, 1011. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Long, M.; Li, X.; Xu, H.; Huang, H.; Gao, Y. Theoretical prediction of electronic structure and carrier mobility in single-walled MoS2 nanotubes. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Marks, T.J.; Hersam, M.C. Mixed-dimensional van der Waals heterostructures. Nat. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wen, X.; Zhang, J.; Wu, T.; Gong, Y.; Zhang, X.; Zhuang, W. Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2 heterostructures. Nat. Commun. 2016, 7, 12512. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, D.; Henck, H.; Avila, J.; Balan, A.; Naylor, C.H.; Patriarche, G.; Asensio, M.C. Band alignment and minigaps in monolayer MoS2-graphene van der Waals heterostructures. Nano Lett. 2016, 16, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Coy Diaz, H.; Avila, J.; Chen, C.; Addou, R.; Asensio, M.C.; Batzill, M. Direct observation of interlayer hybridization and Dirac relativistic carriers in graphene/MoS2 van der Waals heterostructures. Nano Lett. 2015, 15, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-J.; Wang, Q.H.; Son, Y.; Jin, Z.; Blankschtein, D.; Strano, M.S. Tuning On−Off Current Ratio and Field-Effect Mobility in a MoS2−Graphene Heterostructure via Schottky Barrier Modulation. ACS Nano 2014, 8, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, J.T.; Sazaki, G.; Nishikata, S.; Al-Mahboob, A.; Fujikawa, Y.; Nakajima, K.; Sakurai, T. Single-nucleus polycrystallization in thin film epitaxial growth. Phys. Rev. Lett. 2007, 98, 046104. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahboob, A.; Sadowski, J.T.; Nishihara, T.; Fujikawa, Y.; Xue, Q.K.; Nakajima, K.; Sakurai, T. Epitaxial structures of self-organized, standing-up pentacene thin films studied by LEEM and STM. Surf. Sci. 2007, 601, 1304–1310. [Google Scholar] [CrossRef]
- Kelley, T.W.; Baude, P.F.; Gerlach, C.; Ender, D.E.; Muyres, D.; Haase, M.A.; Theiss, S.D. Recent progress in organic electronics: Materials, devices, and processes. Chem. Mater. 2004, 16, 4413–4422. [Google Scholar] [CrossRef]
- Lu, J.; Yeo, P.S.E.; Zheng, Y.; Xu, H.; Gan, C.K.; Sullivan, M.B.; Loh, K.P. Step flow versus mosaic film growth in hexagonal boron nitride. J. Am. Chem. Soc. 2013, 135, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Götzen, J.; Käfer, D.; Wöll, C.; Witte, G. Growth and structure of pentacene films on graphite: Weak adhesion as a key for epitaxial film growth. Phys. Rev. B 2010, 81, 085440. [Google Scholar] [CrossRef]
- Zhang, L.; Roy, S.S.; Hamers, R.J.; Arnold, M.S.; Andrew, T.L. Molecular Orientation-Dependent Interfacial Energetics and Built-in Voltage Tuned by a Template Graphene Monolayer. J. Phys. Chem. C 2014, 119, 45–54. [Google Scholar] [CrossRef]
- Salzmann, I.; Moser, A.; Oehzelt, M.; Breuer, T.; Feng, X.; Juang, Z.Y.; Brillante, A. Epitaxial growth of π-stacked perfluoropentacene on graphene-coated quartz. ACS Nano 2012, 6, 10874–10883. [Google Scholar] [CrossRef] [PubMed]
- Kholmanov, I.N.; Gavioli, L.; Fanetti, M.; Casella, M.; Cepek, C.; Mattevi, C.; Sancrotti, M. Effect of substrate surface defects on the morphology of Fe film deposited on graphite. Surf. Sci. 2007, 601, 188–192. [Google Scholar] [CrossRef]
- Mao, H.Y.; Wang, R.; Wang, Y.; Niu, T.C.; Zhong, J.Q.; Huang, M.Y.; Chen, W. Chemical vapor deposition graphene as structural template to control interfacial molecular orientation of chloroaluminium phthalocyanine. Appl. Phys. Lett. 2011, 99, 093301. [Google Scholar]
- Jariwala, D.; Howell, S.L.; Chen, K.S.; Kang, J.; Sangwan, V.K.; Filippone, S.A.; Hersam, M.C. Hybrid, Gate-Tunable, van der Waals p–n Heterojunctions from Pentacene and MoS2. Nano Lett. 2015, 16, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Furchi, M.M.; Pospischil, A.; Libisch, F.; Burgdörfer, J.; Mueller, T. Photovoltaic effect in an electrically tunable van der Waals heterojunction. Nano Lett. 2014, 14, 4785–4791. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, G.H.; Van Der Zande, A.M.; Chen, W.; Li, Y.; Han, M.; Guo, J. Atomically thin p-n junctions with van der Waals heterointerfaces. Nat. Nanotechnol. 2014, 9, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Seo, J.W.T.; Xu, W.; Smith, J.; Kim, C.H.; Hersam, M.C. Large-area, low-voltage, antiambipolar heterojunctions from solution-processed semiconductors. Nano Lett. 2014, 15, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F. Amplitude shift keying. In Encyclopedia of RF and Microwave Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Mativetsky, J.M.; Wang, H.; Lee, S.S.; Whittaker-Brooks, L.; Loo, Y.L. Face-on stacking and enhanced out-of-plane hole mobility in graphene-templated copper phthalocyanine. Chem. Commun. 2014, 50, 5319–5321. [Google Scholar] [CrossRef] [PubMed]
- Della Pirriera, M.; Puigdollers, J.; Voz, C.; Stella, M.; Bertomeu, J.; Alcubilla, R. Optoelectronic properties of CuPc thin films deposited at different substrate temperatures. J. Phys. D Appl. Phys. 2009, 42, 145102. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J.; Dodabalapur, A. Organic field-effect transistors with high mobility based on copper phthalocyanine. Appl. Phys. Lett. 1996, 69, 3066–3068. [Google Scholar] [CrossRef]
- Xiao, K.; Deng, W.; Keum, J.K.; Yoon, M.; Vlassiouk, I.V.; Clark, K.W.; Li, A.-P.; Kravchenko, I.I.; Gu, G.; Payzant, E.A.; et al. Surface-induced orientation control of CuPc molecules for the epitaxial growth of highly ordered organic crystals on graphene. J. Am. Chem. Soc. 2013, 135, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, S.; Kang, S.J.; Lee, H.; Yi, Y. Impacts of Molecular Orientation on the Hole Injection Barrier Reduction: CuPc/HAT-CN/Graphene. J. Phys. Chem. C 2016, 120, 2292–2298. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Huang, H.; Lyu, L.; Zhang, H.; Cao, N.; Gao, Y. Thickness-dependent air-exposure-induced phase transition of CuPc ultrathin films to well-ordered one-dimensional nanocrystals on layered substrates. J. Phys. Chem. C 2015, 119, 4217–4223. [Google Scholar] [CrossRef]
- Cao, N.-T.; Zhang, L.; Lü, L.; Xie, H.-P.; Huang, H.; Niu, D.-M.; Gao, Y.-L. van der Waals heterostructure about CuPc/MoS2 (0001). Acta Phys. Sin. 2014, 63, 167903. [Google Scholar]
- Pak, J.; Jang, J.; Cho, K.; Kim, T.Y.; Kim, J.K.; Song, Y.; Lee, T. Enhancement of photodetection characteristics of MoS2 field effect transistors using surface treatment with copper phthalocyanine. Nanoscale 2015, 7, 18780–18788. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Deyra, D.M.; Nakajima, K.; Furukawa, Y. Micro-Raman spectroscopy on pentacene thin-film transistors. Mol. Crys. Liq. Crys. 2008, 491, 317–323. [Google Scholar] [CrossRef]
- Chhikara, M.; Pavlica, E.; Matković, A.; Gajić, R.; Bratina, G. Effect of Water Layer at the SiO2/Graphene Interface on Pentacene Morphology. Langmuir 2014, 30, 11681–11688. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Fang, W.; Lee, Y.H.; Araujo, P.T.; Zhang, X.; Rodriguez-Nieva, J.F.; Dresselhaus, M.S. Raman enhancement effect on two-dimensional layered materials: Graphene, h-BN and MoS2. Nano Lett. 2014, 14, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, Y.; Wu, Q.; Xu, R.; Nan, H.; Liu, J.; Shi, Y. Two-dimensional quasi-freestanding molecular crystals for high-performance organic field-effect transistors. Nat. Commun. 2014, 5, 5162. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Pan, Y.; Nan, H.; Gu, S.; Yang, Z.; Wu, B.; Ni, Z. A van der Waals pn heterojunction with organic/inorganic semiconductors. Appl. Phys. Lett. 2015, 107, 183103. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.; Britnell, L.; Wehling, T.O.; Eckmann, A.; Jalil, R.; Belle, B.D.; Novoselov, K.S. Doping mechanisms in graphene-MoS2 hybrids. Appl. Phys. Lett. 2013, 103, 251607. [Google Scholar] [CrossRef]
- Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Zhang, J. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pan, Y.; Shen, Y.; Wang, Z.; Ong, Z.Y.; Xu, T.; Wang, J. Towards intrinsic charge transport in monolayer molybdenum disulfide by defect and interface engineering. Nat. Commun. 2014, 5, 5290. [Google Scholar] [CrossRef] [PubMed]
- Smits, E.C.; Mathijssen, S.G.; Van Hal, P.A.; Setayesh, S.; Geuns, T.C.; Mutsaers, K.A.; Kemerink, M. Bottom-up organic integrated circuits. Nature 2008, 455, 956–959. [Google Scholar] [CrossRef]
- Li, L.; Gao, P.; Wang, W.; Müllen, K.; Fuchs, H.; Chi, L. Growth of ultrathin organic semiconductor microstripes with thickness control in the monolayer precision. Angew. Chem. Int. Ed. 2013, 52, 12530–12535. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, S.G.; Smits, E.C.; van Hal, P.A.; Wondergem, H.J.; Ponomarenko, S.A.; Moser, A.; de Leeuw, D.M. Monolayer coverage and channel length set the mobility in self-assembled monolayer field-effect transistors. Nat. Nanotechnol. 2009, 4, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, F.; Murgia, M.; Levy, P.; Cavallini, M.; Biscarini, F.; de Leeuw, D.M. Spatially correlated charge transport in organic thin film transistors. Phys. Rev. Lett. 2004, 92, 116802. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Papadimitratos, A.; Mayer, A.C.; Malliaras, G.G. Thickness Dependence of Mobility in Pentacene Thin-Film Transistors. Adv. Mater. 2005, 17, 1795–1798. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, Y.; Nan, H.; Yang, Z.; Zhang, Y.; Zhao, H.; Shi, Y. Precise, self-limited epitaxy of ultrathin organic semiconductors and heterojunctions tailored by van der Waals interactions. Nano Lett. 2016, 6, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, S.; Gao, X.; Chen, W.; Wee, A.T.S. Structural and electronic properties of PTCDA thin films on epitaxial graphene. ACS Nano 2009, 3, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Chu, Y.H.; Lu, C.I.; Butler, C.J.; Sankar, R.; Chou, F.C.; Lin, M.T. Organic Monolayer Protected Topological Surface State. Nano Lett. 2015, 15, 6896–6900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Huang, Y.L.; Chen, Y.; Zhao, W.; Eda, G.; Spataru, C.D.; Quek, S.Y. Heterointerface Screening Effects between Organic Monolayers and Monolayer Transition Metal Dichalcogenides. ACS Nano 2016, 10, 2476–2484. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Huang, Y.; Wang, S.; Zhu, M.; Xie, H.; Zhang, L.; Zheng, X.; Xie, Q.; Niu, D.; Gao, Y. Van Der Waals Heterostructures between Small Organic Molecules and Layered Substrates. Crystals 2016, 6, 113. https://doi.org/10.3390/cryst6090113
Huang H, Huang Y, Wang S, Zhu M, Xie H, Zhang L, Zheng X, Xie Q, Niu D, Gao Y. Van Der Waals Heterostructures between Small Organic Molecules and Layered Substrates. Crystals. 2016; 6(9):113. https://doi.org/10.3390/cryst6090113
Chicago/Turabian StyleHuang, Han, Yingbao Huang, Shitan Wang, Menglong Zhu, Haipeng Xie, Lei Zhang, Xiaoming Zheng, Qiliang Xie, Dongmei Niu, and Yongli Gao. 2016. "Van Der Waals Heterostructures between Small Organic Molecules and Layered Substrates" Crystals 6, no. 9: 113. https://doi.org/10.3390/cryst6090113
APA StyleHuang, H., Huang, Y., Wang, S., Zhu, M., Xie, H., Zhang, L., Zheng, X., Xie, Q., Niu, D., & Gao, Y. (2016). Van Der Waals Heterostructures between Small Organic Molecules and Layered Substrates. Crystals, 6(9), 113. https://doi.org/10.3390/cryst6090113