Identification and Crystallization of Penicillin-Binding Protein/β-Lactamase Homolog (Rp46) from Ruegeria Pomeroyi
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Cloning, Expression, and Purification of Rp46
2.2. Biochemical Characterization
2.3. Crystallization
2.4. X-ray Data Collection and Data Processing
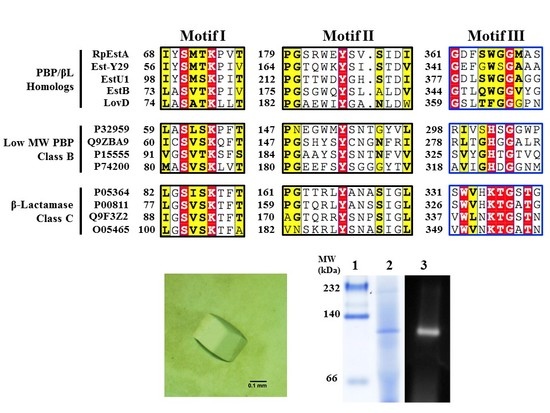
3. Results and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macheboeuf, P.; Contreras-Martel, C.; Job, V.; Dideberg, O.; Dessen, A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol. Rev. 2006, 30, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.D.; Ollis, D.L. α/βHydrolase Fold: An Update. Protein Pept. Lett. 2009, 16, 1137–1148. [Google Scholar] [PubMed]
- Pérez-Llarena, F.J.; Bou, G. Design and Synthesis of Mimics of the T7-loop of FtsZ. Curr. Med. Chem. 2009, 16, 3740–3765. [Google Scholar] [PubMed]
- Meroueh, S.O.; Minasov, G.; Lee, W.; Shoichet, B.K.; Mobashery, S. Structural Aspects for Evolution of β-Lactamases from Penicillin-Binding Proteins. J. Am. Chem. Soc. 2003, 125, 9612–9618. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Barlow, M. Structure-based phylogenies of the serine beta-lactamases. J. Mol. Evol. 2003, 57, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, A.; Schneider, I.; Jungwirth, R.; Sahly, H.; Ullmann, U. A Novel Type of AmpC β-Lactamase, ACC-1, Produced by a Klebsiella pneumoniae Strain Causing Nosocomial Pneumonia. Antimicrob. Agents Chemother. 1999, 43, 1924–1931. [Google Scholar] [PubMed]
- Petersen, E.I.; Valinger, G.; Sölkner, B.; Stubenrauch, G.; Schwab, H. A novel esterase from Burkholderia gladioli which shows high deacetylation activity on cephalosporins is related to beta-lactamases and dd-peptidases. J. Biotechnol. 2001, 89, 11–25. [Google Scholar] [CrossRef]
- Schütte, M.; Fetzner, S. EstA from Arthrobacter nitroguajacolicus Rü61a, a thermo- and solvent-tolerant carboxylesterase related to class C beta-lactamases. Curr. Microbiol. 2007, 54, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Y.; Smith, D.; Konermann, L.; Siu, K.W.; Golemi-Kotra, D. Diversity of penicillin-binding proteins. Resistance factor FmtA of Staphylococcus aureus. J. Biol. Chem. 2007, 282, 35143–35152. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Kovacic, F.; Wilhelm, S.; Jaeger, K.E.; García, M.T.; Ventosa, A.; Mellado, E. Identification of Amino Acids Involved in the Hydrolytic Activity of Lipase LipBL From Marinobacter Lipolyticus. Microbiology 2012, 158, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.H.; Ngo, T.D.; Jang, E.; Kim, S.; Ju, H.; Kim, K.K.; Kim, T.D. Identification, crystallization and preliminary X-ray diffraction analysis of esterase A from Caulobacter crescentus CB15, a family VIII lipolytic enzyme. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ngo, T.D.; Kim, K.K.; Kim, T.D. Characterization, crystallization and preliminary Xray diffraction analysis of an (S)-specific esterase (pfEstA) from Pseudomonas fluorescens KCTC 1767: Enantioselectivity for potential industrial applications. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, S.; Ryu, Y.; Kim, T.D. Identification and characterization of a novel (S)-ketoprofen-specific esterase. Int. J. Biol. Macromol. 2007, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rashamuse, K.; Magomani, V.; Ronneburg, T.; Brady, D. A novel family VIII carboxylesterase derived from a leachate metagenome library exhibits promiscuous beta-lactamase activity on nitrocefin. Appl. Microbiol. Biotechnol. 2009, 83, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, S.J.; Lee, H.S.; Cha, S.S.; Lee, J.H.; Yoon, S.H.; Koo, B.S.; Lee, C.M.; Choi, S.H.; Lee, S.H.; et al. Novel Metagenome-Derived Carboxylesterase That Hydrolyzes β-Lactam Antibiotics. Appl. Environ. Microbiol. 2011, 77, 7830–7836. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, N.; Mathiba, K.; Tsekoa, T.; Steenkamp, P.; Rashamuse, K. Functional Characterisation of a Metagenome Derived Family VIII Esterase with a Deacetylation Activity on β-Lactam Antibiotics. Biochem. Biophys. Res. Commun. 2013, 437, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.F. β-Lactamases: Why and How. J. Med. Chem. 2016, 59, 8207–8220. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.G.; Petersen, E.I.; Schwab, H.; Kratky, C. EstB from Burkholderia gladioli: A novel esterase with a β-lactamase fold reveals steric factors to discriminate between esterolytic and β-lactam cleaving activity. Protein Sci. 2002, 11, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, X.; Pashkov, I.; Sawaya, M.R.; Laidman, J.; Zhang, W.; Cacho, R.; Yeates, T.O.; Tang, Y. Directed evolution and structural characterization of a simvastatin synthase. Chem. Biol. 2009, 16, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.S.; An, Y.J.; Jeong, C.S.; Kim, M.K.; Jeon, J.H.; Lee, C.M.; Lee, H.S.; Kang, S.G.; Lee, J.H. Structural basis for the β-lactamase activity of EstU1, a family VIII carboxylesterase. Proteins 2013, 81, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Ryu, B.H.; Ju, H.; Jang, E.J.; Kim, K.K.; Kim, T.D. Crystallographic analysis and biochemical applications of a novel penicillin-binding protein/β-lactamase homologue from a metagenomic library. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.H.; Ngo, T.D.; Yoo, W.; Lee, S.; Kim, B.Y.; Lee, E.; Kim, K.K.; Kim, T.D. Biochemical and Structural Analysis of a Novel Esterase from Caulobacter crescentus related to Penicillin-Binding Protein (PBP). Sci. Rep. 2016, 6, 37978. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Gonzalez, J.M.; Moran, M.A. Overview of the Marine Roseobacter Lineage. Appl. Environ. Microbiol. 2005, 71, 5665–5677. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Ryu, B.H.; Kim, T.D. Identification, characterization, immobilization of a novel type hydrolase (LmH) from Listeria monocytogenes. Int. J. Biol. Macromol. 2015, 72, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Ryu, B.H.; Shim, H.W.; Ju, H.; Kim, D.W.; Kim, T.D. Adsorption of microbial esterases on Bacillus subtilis-templated cobalt oxide nanoparticles. Int. J. Biol. Macromol. 2014, 65, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E.; Shaw Steward, P.D.; Maeder, D.L.; Blow, D.M. An automated system for micro-batch protein crystallization and screening. J. Appl. Cryst. 1990, 23, 297–302. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Frère, J.M.; Page, M.G. Penicillin-binding proteins: Evergreen drug targets. Curr. Opin. Pharmacol. 2014, 18, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Silvaggi, N.R.; Anderson, J.W.; Brinsmade, S.R.; Pratt, R.F.; Kelly, J.A. The crystal structure of phosphonate-inhibited D-Ala-D-Ala peptidase reveals an analogue of a tetrahedral transition state. Biochemistry 2003, 42, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Peitsaro, N.; Polianskyte, Z.; Tuimala, J.; Pörn-Ares, I.; Liobikas, J.; Speer, O.; Lindholm, D.; Thompson, J.; Eriksson, O. Evolution of a family of metazoan active-site-serine enzymes from penicillin-binding proteins: A novel facet of the bacterial legacy. BMC Evol. Biol. 2008, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Gouet, P.; Robert, X.; Courcelle, E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003, 31, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]




| Space Group | I422 |
|---|---|
| Unit cell parameters (Å) | a = b = 141.26, c = 119.75 |
| Wavelength (Å) | 1.000 |
| Resolution (Å) | 50.00–1.90 (1.93–1.90) |
| Unique reflections | 92,068 (4589) |
| Completeness (%) | 99.9 (100) |
| Redundancy | 7.6 (7.2) |
| Rmerge 1 (%) | 7.4 (34.5) |
| Mean I/σ (I) | 44.1 (7.0) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.H.; Ngo, T.D.; Yoo, W.; Kim, K.K.; Kim, T.D. Identification and Crystallization of Penicillin-Binding Protein/β-Lactamase Homolog (Rp46) from Ruegeria Pomeroyi. Crystals 2017, 7, 6. https://doi.org/10.3390/cryst7010006
Ryu BH, Ngo TD, Yoo W, Kim KK, Kim TD. Identification and Crystallization of Penicillin-Binding Protein/β-Lactamase Homolog (Rp46) from Ruegeria Pomeroyi. Crystals. 2017; 7(1):6. https://doi.org/10.3390/cryst7010006
Chicago/Turabian StyleRyu, Bum Han, Tri Duc Ngo, Wanki Yoo, Kyeong Kyu Kim, and T. Doohun Kim. 2017. "Identification and Crystallization of Penicillin-Binding Protein/β-Lactamase Homolog (Rp46) from Ruegeria Pomeroyi" Crystals 7, no. 1: 6. https://doi.org/10.3390/cryst7010006
APA StyleRyu, B. H., Ngo, T. D., Yoo, W., Kim, K. K., & Kim, T. D. (2017). Identification and Crystallization of Penicillin-Binding Protein/β-Lactamase Homolog (Rp46) from Ruegeria Pomeroyi. Crystals, 7(1), 6. https://doi.org/10.3390/cryst7010006