Separating NaCl and AlCl3·6H2O Crystals from Acidic Solution Assisted by the Non-Equilibrium Phase Diagram of AlCl3-NaCl-H2O(-HCl) Salt-Water System at 353.15 K
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
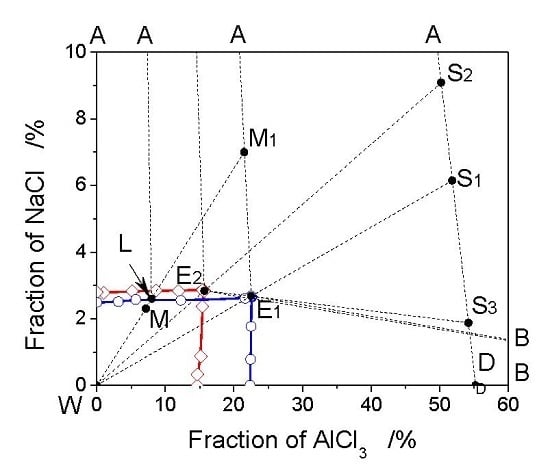
3.1. The Phase Diagram of the AlCl3-NaCl-H2O System
3.2. The Analysis of Crystallization Process Based on Phase Diagrams
3.3. Optimization of Crystallization Process Based on Phase Diagram
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sibanda, V.; Ndlovu, S.; Dombo, G.; Shemi, A.; Rampou, M. Towards the Utilization of Fly Ash as a Feedstock for Smelter Grade Alumina Production: A Review of the Developments. J. Sustain. Metall. 2016, 2, 167–184. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Gong, J.B.; Wang, Y.; Du, S.C.; Dong, W.B.; Yu, B.; Wu, S.G.; Hou, J.; Wang, J.K. Industrial Crystallization in China. Chem. Eng. Technol. 2016, 39, 807–814. [Google Scholar] [CrossRef]
- Lewis, A.; Seckler, M.; Kramer, H.; Van Rosmalen, G. Industrial Crystallization: Fundamentals and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 4–13. [Google Scholar]
- Guo, Y.; Lv, H.; Yang, X.; Cheng, F. AlCl3·6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring. Sep. Purif. Technol. 2015, 151, 177–183. [Google Scholar] [CrossRef]
- Gao, W.C.; Li, Z.B. Solubility of AlCl3·6H2O in the Fe(II) plus Mg plus Ca plus K+Cl+H2O System and its salting-out crystallization with FeCl2. Ind. Eng. Chem. Res. 2013, 52, 14282–14290. [Google Scholar] [CrossRef]
- Wang, J.; Petit, C.; Zhang, X.; Cui, S. Phase Equilibrium Study of the AlCl3-CaCl2-H2O System for the Production of Aluminum Chloride Hexahydrate from Ca-Rich Flue Ash. J. Chem. Eng. Data 2016, 61, 359–369. [Google Scholar] [CrossRef]
- Yuan, M.; Qiao, X.; Yu, J. Phase equilibria of AlCl3 + FeCl3 + H2O, AlCl3 + CaCl2 + H2O, and FeCl3 + CaCl2 + H2O at 298.15 K. J. Chem. Eng. Data 2016, 61, 1749–1755. [Google Scholar] [CrossRef]
- Farelo, F.; Fernandes, C.; Avelino, A. Solubilities for Six Ternary Systems: NaCl+NH4Cl+H2O, KCl+NH4Cl+H2O, NaCl+LiCl+H2O, KCl+LiCl+H2O, NaCl+AlCl3+H2O, and KCl+AlCl3+H2O at T=(298 to 333) K. J. Chem. Eng. Data 2005, 50, 1470–1477. [Google Scholar] [CrossRef]
- Sarkarov, R.A.; Mironova, O.P. Solubility in the AlCl3-LiCl-NaCl-H2O System. Zh. Neorg. Khim. 1990, 35, 280–282. [Google Scholar]
- Ulrich, J.; Frohberg, P. Problems, potentials and future of industrial crystallization. Front. Chem. Sci. Eng. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Kang, Q.; Zhang, J.; Zhang, H.; Yuan, J.; Sha, Z. Non-equilibrium State Salt-forming Phase Diagram: Utilization of Bittern Resource in High Efficiency. Chin. J. Chem. Eng. 2010, 18, 635–641. [Google Scholar] [CrossRef]
- Skiba, G.S.; Sel’kina, Y.A. The NaCl-AlCl3-HCl-H2O System at 25 °С. Russ. J. Inorg. Chem. 2016, 61, 1031–1034. [Google Scholar] [CrossRef]



| Salts | Contents (wt%) | ||||
|---|---|---|---|---|---|
| AlCl3·6H2O | NaCl | Impurities | |||
| Experimental | Theoretical | Experimental | Theoretical | ||
| NaCl | 0 | 0 | 99.12 | 100.00 | 0.88 |
| AlCl3·6H2O (S1) | 90.06 | 93.85 | 5.20 | 6.15 | 4.74 |
| AlCl3·6H2O (S3) | 97.35 | 98.12 | 1.32 | 1.88 | 1.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Zhang, J.; Lv, H.; Guo, Y.; Cheng, W.; Zhao, J.; Cheng, F. Separating NaCl and AlCl3·6H2O Crystals from Acidic Solution Assisted by the Non-Equilibrium Phase Diagram of AlCl3-NaCl-H2O(-HCl) Salt-Water System at 353.15 K. Crystals 2017, 7, 244. https://doi.org/10.3390/cryst7080244
Cheng H, Zhang J, Lv H, Guo Y, Cheng W, Zhao J, Cheng F. Separating NaCl and AlCl3·6H2O Crystals from Acidic Solution Assisted by the Non-Equilibrium Phase Diagram of AlCl3-NaCl-H2O(-HCl) Salt-Water System at 353.15 K. Crystals. 2017; 7(8):244. https://doi.org/10.3390/cryst7080244
Chicago/Turabian StyleCheng, Huaigang, Jianwei Zhang, Huibin Lv, Yanxia Guo, Wenting Cheng, Jing Zhao, and Fangqin Cheng. 2017. "Separating NaCl and AlCl3·6H2O Crystals from Acidic Solution Assisted by the Non-Equilibrium Phase Diagram of AlCl3-NaCl-H2O(-HCl) Salt-Water System at 353.15 K" Crystals 7, no. 8: 244. https://doi.org/10.3390/cryst7080244
APA StyleCheng, H., Zhang, J., Lv, H., Guo, Y., Cheng, W., Zhao, J., & Cheng, F. (2017). Separating NaCl and AlCl3·6H2O Crystals from Acidic Solution Assisted by the Non-Equilibrium Phase Diagram of AlCl3-NaCl-H2O(-HCl) Salt-Water System at 353.15 K. Crystals, 7(8), 244. https://doi.org/10.3390/cryst7080244