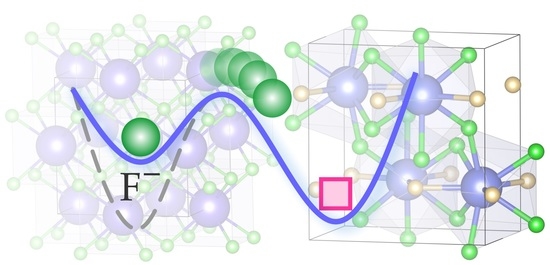
Fluorine Translational Anion Dynamics in Nanocrystalline Ceramics: SrF2-YF3 Solid Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Structure: X-ray Diffraction and F High-Resolution MAS NMR Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vollath, D. Nanomaterials—An Introduction to Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2008; pp. 21–22. [Google Scholar]
- Tuller, H.L. Ionic conduction in nanocrystalline materials. Solid State Ion. 2000, 131, 143–157. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsurekawa, S.; Watanabe, T. A new approach to grain boundary engineering for nanocrystalline materials. Beilstein J. Nanotechnol. 2016, 7, 1829. [Google Scholar] [CrossRef] [PubMed]
- Uitz, M.; Epp, V.; Bottke, P.; Wilkening, M. Ion dynamics in solid electrolytes for lithium batteries. J. Electroceram. 2017, 38, 142–156. [Google Scholar] [CrossRef]
- Heitjans, P.; Indris, S. Diffusion and ionic conduction in nanocrystalline ceramics. J. Phys. Condens. Matter 2003, 15, R1257. [Google Scholar] [CrossRef]
- Prutsch, D.; Breuer, S.; Uitz, M.; Bottke, P.; Langer, J.; Lunghammer, S.; Philipp, M.; Posch, P.; Pregartner, V.; Stanje, B.; et al. Nanostructured Ceramics: Ionic Transport and Electrochemical Activity. Z. Phys. Chem. 2017, 231, 1361–1405. [Google Scholar] [CrossRef]
- Heitjans, P.; Masoud, M.; Feldhoff, A.; Wilkening, M. NMR and impedance studies of nanocrystalline and amorphous ion conductors: Lithium niobate as a model system. Faraday Discuss. 2007, 134, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Epp, V.; Wilkening, M. Motion of Li+ in nanoengineered LiBH4 and LiBH4:Al2O3 comparison with the microcrystalline form. ChemPhysChem 2013, 14, 3706–3713. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, M.; Epp, V.; Feldhoff, A.; Heitjans, P. Tuning the Li diffusivity of poor ionic conductors by mechanical treatment: High Li conductivity of strongly defective LiTaO3 nanoparticles. J. Phys. Chem. C 2008, 112, 9291–9300. [Google Scholar] [CrossRef]
- Düvel, A.; Ruprecht, B.; Heitjans, P.; Wilkening, M. Mixed Alkaline-Earth Effect in the Metastable Anion Conductor Ba1−xCaxF2 (0 ≤ x ≤ 1): Correlating Long-Range Ion Transport with Local Structures Revealed by Ultrafast 19F MAS NMR. J. Phys. Chem. C 2011, 115, 23784–23789. [Google Scholar] [CrossRef]
- Ruprecht, B.; Wilkening, M.; Steuernagel, S.; Heitjans, P. Anion diffusivity in highly conductive nanocrystalline BaF2:CaF2 composites prepared by high-energy ball milling. J. Mater. Chem. 2008, 18, 5412–5416. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Buchinskaya, I.I.; Fedorov, P.P.; Sobolev, B.P. Electrical conductivity of a CaF2-BaF2 nanocomposite. Inorg. Mater. 2008, 44, 189–192. [Google Scholar]
- Maier, J. Ionic conduction in space charge regions. Prog. Solid State Chem. 1995, 23, 171–263. [Google Scholar] [CrossRef]
- Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. Nat. Mater. 2005, 4, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Maier, J. Nano-Ionics: Trivial and Non-Trivial Size Effects on Ion Conduction in Solids. Zeitschrift für Physikalische Chemie 2003, 217, 415–436. [Google Scholar] [CrossRef]
- Maier, J. Nanoionics: Ionic charge carriers in small systems. Phys. Chem. Chem. Phys. 2009, 11, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Puin, W.; Rodewald, S.; Ramlau, R.; Heitjans, P.; Maier, J. Local and overall ionic conductivity in nanocrystalline CaF2. Solid State Ion. 2000, 131, 159–164. [Google Scholar] [CrossRef]
- Blanchard, D.; Nale, A.; Sveinbjörnsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.W.; Vegge, T.; Kentgens, A.P.; de Jongh, P.E. Nanoconfined LiBH4 as a fast lithium ion conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- Sata, N.; Eberman, K.; Eberl, K.; Maier, J. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature 2000, 408, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Jin-Phillipp, N.Y.; Sata, N.; Maier, J.; Scheu, C.; Hahn, K.; Kelsch, M.; Rühle, M. Structures of BaF2-CaF2 heterolayers and their influences on ionic conductivity. J. Chem. Phys. 2004, 120, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Vergentev, T.; Banshchikov, A.; Filimonov, A.; Koroleva, E.; Sokolov, N.; Wurz, M.C. Longitudinal conductivity of LaF3/SrF2 multilayer heterostructures. Sci. Technol. Adv. Mater. 2016, 17, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Šepelak, V.; Düvel, A.; Wilkening, M.; Becker, K.D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, M.; Bork, D.; Indris, S.; Heitjans, P. Diffusion in amorphous LiNbO3 studied by 7Li NMR—Comparison with the nano-and microcrystalline material. Phys. Chem. Chem. Phys. 2002, 4, 3246–3251. [Google Scholar] [CrossRef]
- Wilkening, M.; Indris, S.; Heitjans, P. Heterogeneous lithium diffusion in nanocrystalline Li2O: Al2O3 composites. Phys. Chem. Chem. Phys. 2003, 5, 2225–2231. [Google Scholar] [CrossRef]
- Gleiter, H. Nanoglasses: A new kind of noncrystalline materials. Beilstein J. Nanotechnol. 2013, 4, 517–533. [Google Scholar]
- Chadwick, A.V.; Düvel, A.; Heitjans, P.; Pickup, D.M.; Ramos, S.; Sayle, D.C.; Sayle, T.X.T. X-ray absorption spectroscopy and computer modelling study of nanocrystalline binary alkaline earth fluorides. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2005; Volume 80, p. 012005. [Google Scholar]
- Preishuber-Pflügl, F.; Wilkening, M. Mechanochemically synthesized fluorides: Local structures and ion transport. Dalton Trans. 2016, 45, 8675–8687. [Google Scholar] [CrossRef] [PubMed]
- Preishuber-Pflügl, F.; Bottke, P.; Pregartner, V.; Bitschnau, B.; Wilkening, M. Correlated fluorine diffusion and ionic conduction in the nanocrystalline F− solid electrolyte Ba0.6 La0.4F2.4—19F T1(ρ) NMR relaxation vs. conductivity measurements. Phys. Chem. Chem. Phys. 2014, 16, 9580–9590. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, B.; Wilkening, M.; Feldhoff, A.; Steuernagel, S.; Heitjans, P. High anion conductivity in a ternary non-equilibrium phase of BaF2 and CaF2 with mixed cations. Phys. Chem. Chem. Phys. 2009, 11, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Rongeat, C.; Reddy, M.A.; Witter, R.; Fichtner, M. Nanostructured fluorite-type fluorides as electrolytes for fluoride ion batteries. J. Phys. Chem. C 2013, 117, 4943–4950. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Fichtner, M. Batteries based on fluoride shuttle. J. Mater. Chem. 2011, 21, 17059–17062. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Wissel, K.; Rohrer, J.; Munnangi, A.R.; Clemens, O. LaSrMnO4: Reversible Electrochemical Intercalation of Fluoride Ions in the Context of Fluoride Ion Batteries. Chem. Mater. 2017, 29, 3441–3453. [Google Scholar] [CrossRef]
- Grenier, A.; Porras-Gutierrez, A.G.; Groult, H.; Beyer, K.A.; Borkiewicz, O.J.; Chapman, K.W.; Dambournet, D. Electrochemical reactions in fluoride-ion batteries: Mechanistic insights from pair distribution function analysis. J. Mater. Chem. A 2017, 5, 15700–15705. [Google Scholar] [CrossRef]
- Grenier, A.; Porras-Gutierrez, A.G.; Body, M.; Legein, C.; Chrétien, F.; Raymundo-Pinñero, E.; Dollé, M.; Groult, H.; Dambournet, D. Solid Fluoride Electrolytes and Their Composite with Carbon: Issues and Challenges for Rechargeable Solid State Fluoride-Ion Batteries. J. Phys. Chem. C 2017, 121, 24962–24970. [Google Scholar] [CrossRef]
- Ritter, B.; Krahl, T.; Scholz, G.; Kemnitz, E. Local Structures of Solid Solutions Sr1−x Yx F2+x (x = 0…0.5) with Fluorite Structure Prepared by Sol-Gel and Mechanochemical Syntheses. J. Phys. Chem. C 2016, 120, 8992–8999. [Google Scholar]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten Ges. Wiss. Göttingen 1918, 2, 98. [Google Scholar]
- Düvel, A.; Wegner, S.; Efimov, K.; Feldhoff, A.; Heitjans, P.; Wilkening, M. Access to metastable complex ion conductors via mechanosynthesis: Preparation, microstructure and conductivity of (Ba, Sr) LiF3 with inverse perovskite structure. J. Mater. Chem. 2011, 21, 6238–6250. [Google Scholar] [CrossRef]
- Indris, S.; Bork, D.; Heitjans, P. Nanocrystalline oxide ceramics prepared by high-energy ball milling. J. Mater. Synth. Proc. 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Sadoc, A.; Body, M.; Legein, C.; Biswal, M.; Fayon, F.; Rocquefelte, X.; Boucher, F. NMR parameters in alkali, alkaline earth and rare earth fluorides from first principle calculations. Phys. Chem. Chem. Phys. 2011, 13, 18539–18550. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Düvel, A.; Wilkening, M.; Uecker, R.; Wegner, S.; Šepelak, V.; Heitjans, P. Mechanosynthesized nanocrystalline BaLiF3: The impact of grain boundaries and structural disorder on ionic transport. Phys. Chem. Chem. Phys. 2010, 12, 11251–11262. [Google Scholar] [CrossRef] [PubMed]
- Breuer, S.; Prutsch, D.; Ma, Q.; Epp, V.; Preishuber-Pflügl, F.; Tietz, F.; Wilkening, M. Separating bulk from grain boundary Li ion conductivity in the sol-gel prepared solid electrolyte Li1.5Al0.5Ti1.5(PO4)3. J. Mater. Chem. A 2015, 3, 21343–21350. [Google Scholar] [CrossRef]
- Dyre, J.C.; Maass, P.; Roling, B.; Sidebottom, D.L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72, 046501. [Google Scholar] [CrossRef]
- Sakellis, I. On the origin of time-temperature superposition in disordered solids. Appl. Phys. Lett. 2011, 98, 072904. [Google Scholar] [CrossRef]
- Jonscher, A. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Funke, K.; Cramer, C.; Wilmer, D. Diffusion in Condensed Matter, 2nd ed.; Heitjans, P., Kärger, J., Eds.; Springer: Berlin, Germany, 2005; Chapter 21; pp. 859–893. [Google Scholar]
- Bollmann, W.; Görlich, P.; Hauk, W.; Mothes, H. Ionic conduction of pure and doped CaF2 and SrF2 crystals. Phys. Status Solidi (A) 1970, 2, 157–170. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Sobolev, B.P.; Krivandina, E.A.; Zhmurova, Z.I. Optimization of single crystals of solid electrolytes with tysonite-type structure (LaF3) for conductivity at 293 K: 2. Nonstoichiometric phases R1−yMyF3−y (R = La-Lu, Y; M = Sr, Ba). Cryst. Rep. 2015, 60, 123. [Google Scholar]
- Breuer, S.; Wilkening, M. Mismatch in cation size causes rapid anion dynamics in solid electrolytes: The role of the Arrhenius pre-factor. Dalton Trans. 2018, in press. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuer, S.; Stanje, B.; Pregartner, V.; Lunghammer, S.; Hanzu, I.; Wilkening, M. Fluorine Translational Anion Dynamics in Nanocrystalline Ceramics: SrF2-YF3 Solid Solutions. Crystals 2018, 8, 122. https://doi.org/10.3390/cryst8030122
Breuer S, Stanje B, Pregartner V, Lunghammer S, Hanzu I, Wilkening M. Fluorine Translational Anion Dynamics in Nanocrystalline Ceramics: SrF2-YF3 Solid Solutions. Crystals. 2018; 8(3):122. https://doi.org/10.3390/cryst8030122
Chicago/Turabian StyleBreuer, Stefan, Bernhard Stanje, Veronika Pregartner, Sarah Lunghammer, Ilie Hanzu, and Martin Wilkening. 2018. "Fluorine Translational Anion Dynamics in Nanocrystalline Ceramics: SrF2-YF3 Solid Solutions" Crystals 8, no. 3: 122. https://doi.org/10.3390/cryst8030122
APA StyleBreuer, S., Stanje, B., Pregartner, V., Lunghammer, S., Hanzu, I., & Wilkening, M. (2018). Fluorine Translational Anion Dynamics in Nanocrystalline Ceramics: SrF2-YF3 Solid Solutions. Crystals, 8(3), 122. https://doi.org/10.3390/cryst8030122