Design and Crystal Structures of Two New Compounds Fused with 3,4,5-Trimethoxybenzyl Group and 6,10-Dioxaspiro Group
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Measuremints
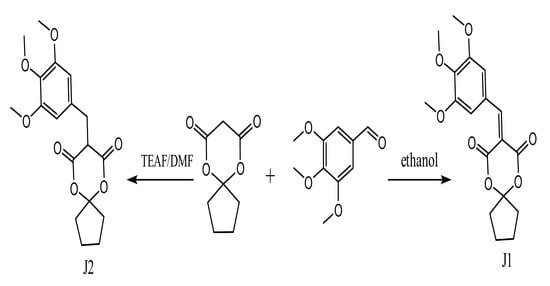
2.2. Preparation of J1 and J2
2.3. Crystallography
3. Results and Discussion
3.1. Crystal Structures
3.2. IR Spectra
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chavan, S.R.; Gavale, K.S.; Khan, A.; Joshi, R.; Kumbhar, N.; Chakravarty, D.; Dhavale, D.D. Iminosugars spiro-linked with morpholine-fused 1,2,3-triazole: Synthesis, conformational analysis, glycosidase inhibitory activity, antifungal assay, and docking studies. ACS Omega 2017, 2, 7203–7218. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bharadwaj, M.; Kumar, A.; Mehrotra, R. Spiro-oxindoles as a promising class of small molecule inhibitors of p53–MDM2 interaction useful in targeted cancer therapy. Top. Curr. Chem. 2017, 375, 3. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Huang, L.; Teng, D.W. Aspirocyclic oxindole analogue: Synthesis and antitumor activities. Chin. Chem. Lett. 2011, 22, 1009–1012. [Google Scholar] [CrossRef]
- Yu, B.; Shi, X.J.; Qi, P.P.; Yu, D.Q.; Liu, H.M. Design, synthesis and biological evaluation of novel steroidal spiro-oxindoles as potent antiproliferative agents. J. Steroid Biochem. Mol. Biol. 2014, 14, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Lombe, B.K.; Bruhn, T.; Feineis, D.; Mudogo, V.; Brun, R.; Bringmann, G. Antiprotozoal spirombandakamines A1 and A2, fused naphthylisoquinoline dimers from a congolese ancistrocladus. Plant. Org. Lett. 2017, 19, 6740–6743. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Singh, R.; Saini, D. Ionic liquid-mediated three-component synthesis of fluorinated spiro-thiazine devivatives and their antimycobacterial and DNA cleavage activities. J. Chem. Sci. Vol. 2013, 125, 1045–1053. [Google Scholar] [CrossRef]
- Takase, K.; Noguchi, K.; Nakano, K. Circularly polarized luminescence from chiral spiro molecules: Synthesis and optical properties of 10, 10’-Spirobi(indeno[1,2-b][1]benzothiophene) derivatives. Org. Lett. 2017, 19, 5082–5085. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Lim, C.S.; Kim, H.M.; Ghosh, K. New six-membered pH-Insensitive rhodamine spirocycle in selective sensing of Cu2+ through C−C bond cleavage and its application in cell imaging. ACS Omega 2017, 2, 8167–8176. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ku, S.Y.; Wong, K.T.; Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem. Commun. 2012, 48, 9580–9582. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Kiriyama, K.; Ieki, K.; Sasai, H. A bifunctional spiro-type organocatalyst with high enantiocontrol: Application to the aza-Morita–Baylis–Hillman reactions. Chem. Commun. 2011, 47, 9227–9229. [Google Scholar] [CrossRef] [PubMed]
- Doddi, S.; Narayanaswamy, K.; Ramakrishna, B.; Singh, S.P.; Banga, P.R. Synthesis and spectroscopic investigation of diketopyrrolopyrrole-spiropyran dyad for fluorescent switch application. J. Fluoresc. 2016, 26, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhou, L.H.; Lv, L.; Zhao, X.; Hao, L.Y. Multi-stimuli-responsive poly(NIPA-co-HEMA-co-NVP) with spironaphthoxazine hydrogel for optical data storage application. Colloid Polym. Sci. 2016, 294, 1623–1632. [Google Scholar] [CrossRef]
- Zeng, W.L. 5,5-[(2,4-Dichlorophenyl)methylene]bis(2,2-dimethyl-1,3dioxane-4,6-dione). Acta Cryst. 2011, 67, o1894. [Google Scholar]
- Jiang, J.H.; Zeng, W.L. Synthesis and Crystal Structures of Two New Oxaspirocyclic Compounds. Crystals 2016, 6, 134. [Google Scholar] [CrossRef]
- Zeng, W.L.; Liu, H.L. Synthesis and Crystal Structure of 3-((m-Tolylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione. Asian J. Chem. 2014, 26, 4356–4358. [Google Scholar]
- Zeng, W.L.; Jiang, J.H. Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds. Crystals 2016, 6, 69. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]




| Compounds | J1 | J2 |
|---|---|---|
| Formula | C18 H20 O7 | C18 H22 O7 |
| CCDC No. | 1567472 | 1567470 |
| Color/shape | red/block | Red/needle |
| Mr | 348.34 | 350.36 |
| Crystal System, Space group | Triclinic, P-1 | Triclinic, P-1 |
| a, b, c (Å) | 5.4056(11), 11.465(2), 13.502(3) | 8.8778(18), 9.5285(19), 11.082(2) |
| α, β, γ (°) | 92.06(3), 91.35(3), 93.50(3) | 89.69(3), 67.38(3), 81.68(3) |
| Crystal Size (mm) | 0.24 × 0.16 × 0.12 | 0.14 × 0.10 × 0.06 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| θ Ranges (°) | 3.02–27.48 | 3.02–27.48 |
| V(Å3) | 3135.5(2) | 854.9(3) |
| Z | 2 | 2 |
| F(000) | 368 | 372 |
| D/g·cm−3 | 1.386 | 1.361 |
| μ/ mm−1 | 0.107 | 0.105 |
| −h, h/−k, k/−l, l | −7:6; −14:14; −17:17 | −11:11; −10:12 ; −14:14 |
| Total, unique and [I > 2σ(I)] reflections | 8107, 3785, 1786 | 8389, 3894, 2870 |
| No. of reflections, restraints, parameters | 3785, 0, 226 | 3894, 0, 226 |
| R(int) | 0.0312 | 0.0261 |
| R, wR, S | 0.0651, 0.1847, 1.129 | 0.0578, 0.1682, 1.105 |
| (∆ρ)max, (∆ρ)min (e/Å3) | 0.595, −0.427 | 0.630, −0.334 |
| J1 | J2 | |
|---|---|---|
| Bond | Dist. | Dist. |
| C(8)–C(10) | 1.445(4) | 1.516(2) |
| C(10)–C(12) | 1.359(4) | 1.535(2) |
| C(8)–C(7) | 1.407(4) | 1.380(3) |
| C(9)–C(8) | 1.394(4) | 1.391(2) |
| O(4)–C(11) | 1.206(4) | 1.193(2) |
| O(5)–C(13) | 1.197(4) | 1.199(2) |
| Angle | (°) | (°) |
| C(12)–C(10)–C(8) | 137.3(3) | 114.13(13) |
| C(9)–C(8)–C(7) | 119.1(3) | 119.28(15) |
| C(9)–C(8)–C(10) | 126.7(3) | 122.19(15) |
| C(7)–C(8)–C(10) | 114.2(2) | 118.51(16) |
| C(10)–C(12)–C(13) | 128.22(15) | 109.48(13) |
| C(10)–C(12)–C(11) | 114.83(14) | 113.71(15) |
| C(13)–C(12)–C(11) | 116.91(14) | 112.53(13) |
| D–H···A | Symmetry | D–H(Å) | H···A(Å) | D···A(Å) | ∠D–H···A (°) |
|---|---|---|---|---|---|
| C(2)–H(2B)···O(3) (J1) | intra | 0.9600 | 2.4481 | 3.0097(2) | 117.13 |
| C(9)–H(9A)···O(4) (J1) | intra | 0.9300 | 2.1532 | 2.9287(2) | 140.18 |
| C(10)–H(10A)···O(5) (J1) | intra | 0.9300 | 2.3080 | 2.7619(2) | 109.55 |
| C(1)–H(1A)···O(4) (J1) | −1 − x,2 − y,−z | 0.9600 | 2.5946 | 3.4547(2) | 149.25 |
| C(7)–H(7A)···O(5) (J1) | 1 − x,1 − y,−z | 0.9300 | 2.3911 | 3.3017(3) | 166.21 |
| C(18)–H(18B)···O(1) (J1) | −x,2 − y,−z | 0.9700 | 2.5597 | 3.4643(2) | 155.20 |
| C(10)–H(10B)···O(4) (J2) | intra | 0.9700 | 2.4393 | 2.843(2) | 104.60 |
| C(7)–H(7A)···O(5) (J2) | intra | 0.9300 | 2.5840 | 3.118(2) | 116.97 |
| C(2)–H(2B)···O(3) (J2) | intra | 0.9599 | 2.3090 | 2.929(3) | 121.63 |
| C(12)–H(12A)···O(1) (J2) | 1 − x,−y,1 − z | 0.9804 | 2.3854 | 3.300(2) | 154.88 |
| C(9)–H(9A)····O(4) (J2) | 1 − x,−1 − y,1 − z | 0.9299 | 2.5723 | 3.170(2) | 122.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Wang, X.; Jiang, J. Design and Crystal Structures of Two New Compounds Fused with 3,4,5-Trimethoxybenzyl Group and 6,10-Dioxaspiro Group. Crystals 2018, 8, 146. https://doi.org/10.3390/cryst8040146
Zeng W, Wang X, Jiang J. Design and Crystal Structures of Two New Compounds Fused with 3,4,5-Trimethoxybenzyl Group and 6,10-Dioxaspiro Group. Crystals. 2018; 8(4):146. https://doi.org/10.3390/cryst8040146
Chicago/Turabian StyleZeng, Wulan, Xia Wang, and Jinhe Jiang. 2018. "Design and Crystal Structures of Two New Compounds Fused with 3,4,5-Trimethoxybenzyl Group and 6,10-Dioxaspiro Group" Crystals 8, no. 4: 146. https://doi.org/10.3390/cryst8040146
APA StyleZeng, W., Wang, X., & Jiang, J. (2018). Design and Crystal Structures of Two New Compounds Fused with 3,4,5-Trimethoxybenzyl Group and 6,10-Dioxaspiro Group. Crystals, 8(4), 146. https://doi.org/10.3390/cryst8040146