Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization
Abstract
:1. Introduction
2. Materials and Method
3. Results and Discussion
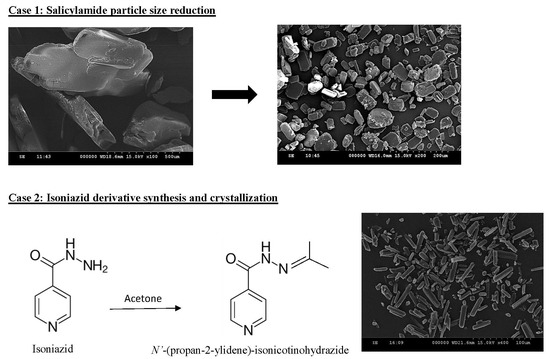
3.1. Particle Size Reduction of Salicylamide
3.2. Synthesis and Crystallization of N′-(Propan-2-ylidene)-isonicotinohydrazide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From form to function: Crystallization of active pharmaceutical ingredients. AIChE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Wong, S.Y.; Chen, J.; Forte, L.E.; Myerson, A.S. Compact crystallization, filtration, and drying for the production of active pharmaceutical ingredients. Org. Process Res. Dev. 2013, 17, 684–692. [Google Scholar] [CrossRef]
- Tung, H.H. Industrial perspectives of pharmaceutical crystallization. Org. Process Res. Dev. 2013, 17, 445–454. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization processes in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Pfund, L.Y.; Price, C.P.; Frick, J.J.; Matzger, A.J. Controlling pharmaceutical crystallization with designed polymeric heteronuclei. J. Am. Chem. Soc. 2015, 137, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Hezave, A.Z.; Esmaeilzadeh, F. Crystallization of micro particles of sulindac using rapid expansion of supercritical solution. J. Cryst. Growth 2010, 312, 3373–3383. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.J.; Zhou, W.; Chen, S.B.; Chen, S.L. Recrystallization of puerarin using the supercritical fluid antisolvent process. J. Cryst. Growth 2012, 340, 142–148. [Google Scholar] [CrossRef]
- Weber, C.C.; Kulkarni, S.A.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. The use of cooling crystallization in an ionic liquid system for the purification of pharmaceuticals. Cryst. Growth Des. 2015, 15, 4946–4951. [Google Scholar] [CrossRef]
- Horstman, E.M.; Goyal, S.; Pawate, A.; Lee, G.; Zhang, G.G.Z.; Gong, Y.; Kenis, P.J.A. Crystallization optimization of pharmaceutical solid forms with X-ray compatible microfluidic platforms. Cryst. Growth Des. 2015, 15, 1201–1209. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 23, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Spray sonocrystallization. Cryst. Growth Des. 2015, 15, 1564–1567. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.S.; Gogate, P.R. Intensified oxalic acid crystallization using ultrasonic reactors: Understanding effect of operating parameters and type of ultrasonic reactor. Ultrason. Sonochem. 2017, 39, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Khan, A.; Bruce, L.M.; Forbes, C.; O’Leary, R.L.; Price, C.J. The effect of ultrasound on the crystallization of paracetamol in the presence of structurally similar impurities. Crystals 2017, 7, 294. [Google Scholar] [CrossRef]
- Ike, Y.; Hirasawa, I. Polymorph control of L-ArgHCl on antisolvent crystallization by ultrasonic irradiation. Chem. Eng. Technol. 2017, 40, 1318–1322. [Google Scholar] [CrossRef]
- Gielen, B.; Jordens, J.; Thomassen, L.C.J.; Braeken, L.; Gerven, T.V. Agglomeration control during ultrasonic crystallization of an active pharmaceutical ingredient. Crystals 2017, 7, 40. [Google Scholar] [CrossRef]
- Gandhi, P.J.; Murthy, Z.V.P.; Pati, R.K. Optimization of process parameters by Taguchi robust design method for the development of nano-crystals of sirolimus using sonication based crystallization. Cryst. Res. Technol. 2012, 47, 53–72. [Google Scholar] [CrossRef]
- Li, J.; Bao, Y.; Wang, J. Effects of sonocrystallization on the crystal size distribution of cloxacillin benzathine crystals. Chem. Eng. Technol. 2013, 36, 1341–1346. [Google Scholar] [CrossRef]
- Crawford, D.E. Solvent-free sonochemistry: Sonochemical organic synthesis in the absence of a liquid medium. Beilstein J. Org. Chem. 2017, 13, 1850–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.S.; Liao, C.Y.; Jheng, W.D. Particle size control and crystal habit modification of phenacetin using ultrasonic crystallization. Chem. Eng. Technol. 2015, 38, 181–186. [Google Scholar] [CrossRef]
- Su, C.S.; Wu, P.Y.; Jheng, W.D. Recrystallization of phenacetin and sulfathiazole using the sonocrystallization process. J. Taiwan Inst. Chem. Eng. 2016, 59, 106–112. [Google Scholar] [CrossRef]
- Kuo, P.H.; Zhang, B.C.; Su, C.S.; Liu, J.J.; Sheu, M.T. Application of two-level factorial design to investigate the effect of process parameters on the sonocrystallization of sulfathiazole. J. Cryst. Growth 2017, 471, 8–14. [Google Scholar] [CrossRef]
- Manin, A.N.; Voronin, A.P.; Manin, N.G.; Vener, M.V.; Shishkina, A.V.; Lermontov, A.S.; Perlovich, G.L. Salicylamide cocrystals: Screening, crystal structure, sublimation thermodynamics, dissolution, and solid-state DFT calculations. J. Phys. Chem. B 2014, 118, 6803–6814. [Google Scholar] [CrossRef] [PubMed]
- Su, C.S.; Chen, Y.P. Recrystallization of salicylamide using a batch supercritical antisolvent process. Chem. Eng. Technol. 2005, 28, 1177–1181. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Zhang, S.; Zhao, F.; Gao, C.; Feng, L.S.; Lv, Z.S.; Xu, Z.; Wu, X. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Lemmerer, A.; Bernstein, J.; Kahlenberg, V. Covalent assistance in supramolecular synthesis: In situ modification and masking of the hydrogen bonding functionality of the supramolecular reagent isoniazid in co-crystals. CrystEngComm 2011, 13, 5692–5708. [Google Scholar] [CrossRef]
- Oruganti, M.; Khade, P.; Das, U.K.; Trivedi, D.R. The hierarchies of hydrogen bonds in salts/cocrystals of isoniazid and its schiff base—A case study. RSC Adv. 2016, 6, 15868–15876. [Google Scholar] [CrossRef]
- Nordström, F.L.; Rasmuson, Å.C. Solubility and melting properties of salicylamide. J. Chem. Eng. Data 2006, 51, 1775–1777. [Google Scholar] [CrossRef]
- Dhumal, R.S.; Biradar, S.V.; Paradkar, A.R.; York, P. Particle engineering using sonocrystallization: Salbutamol sulphate for pulmonary delivery. Int. J. Pharm. 2009, 368, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Narducci, O.; Jones, A.G.; Kougoulos, E. An assessment of the use of ultrasound in the particle engineering of micrometer-scale adipic acid crystals. Cryst. Growth Des. 2011, 11, 1742–1749. [Google Scholar] [CrossRef]











| Compound | CAS. No. | Formula | Mw (g/mol) | Supplier | Purity (%) |
|---|---|---|---|---|---|
| Acetone | 67-64-1 | C3H6O | 58.08 | Sigma-Aldrich | 99.8 |
| Isoniazid | 54-85-3 | C6H7N3O | 137.14 | Sigma-Aldrich | 99 |
| Methanol | 67-56-1 | CH3OH | 32.04 | Sigma-Aldrich | 99.8 |
| Salicylamide | 65-45-2 | C7H7NO2 | 137.14 | Sigma-Aldrich | 99 |
| Experiment No. | Intensity (%) | Duration (%) | Concentration (mg/mL) | Cooling Rate (°C/h) | Mean Size (μm) | S.D a (μm) |
|---|---|---|---|---|---|---|
| Ori | --- | --- | --- | --- | 595.0 | 178.4 |
| 1 | 30 | 30 | 170 | 20 | 45.6 | 18.2 |
| 2 | 10 | 30 | 170 | 20 | 68.5 | 30.4 |
| 3 | 50 | 30 | 170 | 20 | 39.9 | 13.3 |
| 4 | 30 | 10 | 170 | 20 | 80.5 | 26.7 |
| 5 | 30 | 50 | 170 | 20 | 38.4 | 13.7 |
| 6 | 30 | 30 | 140 | 20 | 53.8 | 17.0 |
| 7 | 30 | 30 | 200 | 20 | 59.6 | 22.3 |
| B1 b | 0 | 0 | 170 | 20 | 432.5 | 176.5 |
| Experiment No. | Intensity (%) | Duration (%) | Concentration (mg/mL) | Cooling Rate (°C/h) | Mean Size (μm) | S.D a (μm) |
|---|---|---|---|---|---|---|
| 8 | 30 | 30 | 25 | 20 | 27.6 | 8.7 |
| 9 | 10 | 30 | 25 | 20 | 73.2 | 43.1 |
| 10 | 50 | 30 | 25 | 20 | 31.4 | 10.2 |
| 11 | 30 | 10 | 25 | 20 | 31.4 | 12.3 |
| 12 | 30 | 50 | 25 | 20 | 23.7 | 8.1 |
| 13 | 30 | 30 | 35 | 20 | 27.7 | 10.3 |
| 14 | 30 | 30 | 15 | 20 | 19.4 | 7.9 |
| B2 b | 0 | 0 | 25 | 20 | 78.1 | 41.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.-Y.; Yen, S.-K.; Hu, W.-S.; Huang, Y.-Z.; Yang, T.-M.; Su, C.-S. Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals 2018, 8, 249. https://doi.org/10.3390/cryst8060249
Yang Z-Y, Yen S-K, Hu W-S, Huang Y-Z, Yang T-M, Su C-S. Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals. 2018; 8(6):249. https://doi.org/10.3390/cryst8060249
Chicago/Turabian StyleYang, Zhen-Yu, Shih-Kuo Yen, Wei-Syun Hu, Yu-Zhe Huang, Tsung-Mao Yang, and Chie-Shaan Su. 2018. "Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization" Crystals 8, no. 6: 249. https://doi.org/10.3390/cryst8060249
APA StyleYang, Z.-Y., Yen, S.-K., Hu, W.-S., Huang, Y.-Z., Yang, T.-M., & Su, C.-S. (2018). Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals, 8(6), 249. https://doi.org/10.3390/cryst8060249