Recrystallization and Production of Spherical Submicron Particles of Sulfasalazine Using a Supercritical Antisolvent Process
Abstract
:1. Introduction
2. Materials and Methods
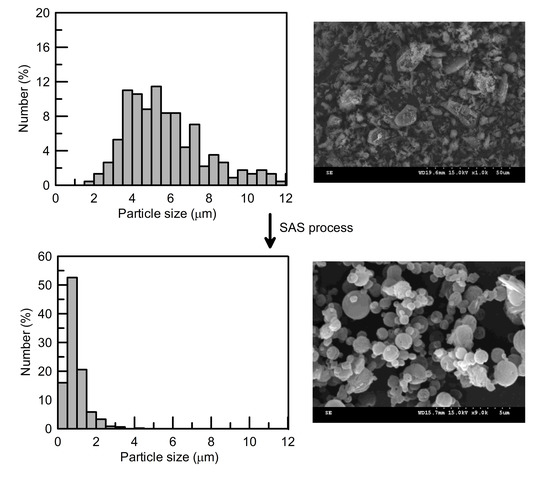
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kawakami, K. Modification of physicochemical characteristics of active pharmaceutical ingredients and application of supersaturatable dosage forms for improving bioavailability of poorly absorbed drugs. Adv. Drug Deliver. Rev. 2012, 64, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Jog, R.; Burgess, D.J. Pharmaceutical amorphous nanoparticles. J. Pharm. Sci. 2017, 106, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Dantuluri, A.K.R.; Perumalla, S.R.; Sun, C.C.; Bansal, A.K. Effect of crystal habit on intrinsic dissolution behavior of celecoxib due to differential wettability. Cryst. Growth Des. 2014, 14, 5283–5292. [Google Scholar] [CrossRef]
- Chattoraj, S.; Sun, C.C. Crystal and particle engineering strategies for improving powder compression and flow properties to enable continuous tablet manufacturing by direct compression. J. Pharm. Sci. 2018, 107, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, N.; Parker, C.R.; Hagen, S.U.; Bond, A.D.; Rantanen, J. Role of solvent selection on crystal habit of 5-aminosalicylic acid-combined experimental and computational approach. J. Pharm. Sci. 2018, 107, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Fahim, T.K.; Zaidul, I.S.M.; Abu Bakar, M.R.; Salim, U.M.; Awang, M.B.; Sahena, F.; Jalal, K.C.A.; Sharif, K.M.; Sohrab, M.H. Particle formation and micronization using non-conventional techniques-review. Chem. Eng. Process. 2014, 86, 47–52. [Google Scholar] [CrossRef]
- Wu, J.J.; Shen, L.Y.; Yin, M.C.; Cheng, Y.S. Supercritical carbon dioxide anti-solvent micronization of lycopene extracted and chromatographic purified from Momordica charantia L. aril. J. Taiwan Inst. Chem. Eng. 2017, 80, 64–70. [Google Scholar] [CrossRef]
- Wu, H.T.; Yang, C.P.; Huang, S.C. Dissolution enhancement of indomethacin-chitosan hydrochloride composite particles produced using supercritical assisted atomization. J. Taiwan Inst. Chem. Eng. 2016, 67, 98–105. [Google Scholar] [CrossRef]
- Jung, J.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Abuzara, S.M.; Hyuna, S.M.; Kim, J.H.; Park, H.J.; Kim, M.S.; Park, J.S.; Hwang, S.J. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int. J. Pharm. 2018, 538, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Cheng, Y.; Wang, Z.; Huang, D.; Miao, H.; Zhang, Y. Preparation and characterization of baicalein powder micronized by the SEDS process. J. Supercrit. Fluids 2015, 104, 177–182. [Google Scholar] [CrossRef]
- Jia, J.; Wang, W.; Gao, Y.; Zhao, Y. Controlled morphology and size of curcumin using ultrasound in supercritical CO2 antisolvent. Ultrason. Sonochem. 2015, 27, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Adami, R.; Campardelli, R.; Della Porta, G.; De Marco, I.; Scognamiglio, M. Supercritical fluids based techniques to process pharmaceuticalproducts difficult to micronize: palmitoylethanolamide. J. Supercrit. Fluids 2015, 102, 24–31. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Masmoudi, Y.; Djerafi, R.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.; Badens, E. Particle design applied to quercetin using supercritical anti-solvent techniques. J. Supercrit. Fluids 2015, 105, 119–127. [Google Scholar] [CrossRef]
- Montes, A.; Pereyra, C.; Martinez de la Ossa, E.J. Screening design of experiment applied to the supercritical antisolvent precipitation of quercetin. J. Supercrit. Fluids. 2015, 104, 10–18. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Higashiura, T.; Kanda, H.; Goto, M. Effect of solvent on nanoparticle production of β-carotene by a supercritical antisolvent process. Chem. Eng. Technol. 2016, 39, 1771–1777. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Generation of microparticles of ellagic acid by supercritical antisolvent process. J. Supercrit. Fluids 2016, 116, 101–110. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, W.; Chen, Z.; Wang, Z.; Huang, D. Micronization of etoposide using solution-enhanced dispersion bysupercritical CO2. J. Supercrit. Fluids 2016, 115, 10–16. [Google Scholar] [CrossRef]
- Kim, D.C.; Yeo, S.D. Modification of indomethacin crystals using supercritical and aqueous antisolvent crystallizations. J. Supercrit. Fluids 2016, 108, 96–103. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Mangiferin nanoparticles precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2016, 112, 44–50. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Precipitation of submicron particles of rutin using supercritical antisolvent process. J. Supercrit. Fluids 2016, 118, 1–10. [Google Scholar] [CrossRef]
- Yoon, T.J.; Son, W.S.; Park, H.J.; Seo, B.; Kim, T.; Lee, Y.W. Tetracycline nanoparticles precipitation using supercritical and liquid CO2 as antisolvents. J. Supercrit. Fluids 2016, 107, 51–60. [Google Scholar] [CrossRef]
- Jia, J.; Wang, J.; Zhang, K.; Zhou, D.; Ge, F.; Zhao, Y. Aescin nanoparticles prepared using SEDS: Composition stability and dissolution enhancement. J. Supercrit. Fluids 2017, 130, 267–272. [Google Scholar] [CrossRef]
- Padrela, L.; Zeglinski, J.; Ryan, K.M. Insight into the role of additives in controlling polymorphic outcome: A CO2-antisolvent crystallization process of carbamazepine. Cryst. Growth Des. 2017, 17, 4544–4553. [Google Scholar] [CrossRef]
- Kurniawansyah, F.; Quachie, L.; Mammucari, R.; Foster, N.R. Improving the dissolution properties of curcumin using dense gas antisolvent technology. Int. J. Pharm. 2017, 521, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lin, Q.; Huang, Y.; Guan, G.; Jiang, Y. Tailoring the particle microstructures of gefitinib by supercritical CO2 antisolvent process. J. CO2 Utiliz. 2017, 20, 43–51. [Google Scholar] [CrossRef]
- Kudryashova, E.V.; Deygen, I.M.; Sukhoverkov, K.V.; Filatova, L.Y.; Klyachko, N.L.; Vorobei, A.M.; Pokrovskiy, O.I.; Ustinovich, K.B.; Parenago, O.O.; Antonov, E.N.; et al. Micronization of levofloxacin by supercritical antisolvent precipitation. Russ. J. Phys. Chem. B 2016, 10, 1201–1210. [Google Scholar] [CrossRef]
- Miao, H.; Chen, Z.; Xu, W.; Wang, W.; Song, Y.; Wang, Z. Preparation and characterization of naringenin microparticles via a supercritical anti-solvent process. J. Supercrit. Fluids 2018, 131, 19–25. [Google Scholar] [CrossRef]
- Chen, H.H.; Su, C.S. Recrystallizing primidone through supercritical antisolvent precipitation. Org. Process Res. Dev. 2016, 20, 878–887. [Google Scholar] [CrossRef]
- Ciou, J.M.; Wang, B.C.; Su, C.S.; Liu, J.J.; Sheu, M.T. Measurement of solid solubility of warfarin in supercritical carbon dioxide and recrystallization study using supercritical antisolvent process. Adv. Powder Technol. 2018, 29, 479–487. [Google Scholar] [CrossRef]
- Wu, W.Y.; Su, C.S. Modification of solid-state property of sulfasalazine by using the supercritical antisolvent process. J. Cryst. Growth 2017, 460, 59–66. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. Dependence of SAS particle morphologies on the ternary phase equilibria. J. Supercrit. Fluids 2017, 130, 273–281. [Google Scholar] [CrossRef]

| Valve Description | Component Description | |
|---|---|---|
| A: Back pressure regulator | 1: CO2 cylinder | 8: Filter |
| B: Check valve | 2: Cooler | 9: Pressure gauge |
| C: Needle valve | 3: HPLC pump | 10: Heating jacket |
| D: Ball valve | 4: Pressure transducer | 11: Solution reservoir |
| E: Micrometering valve | 5: Thermometer | 12: Heating tape |
| – | 6: Nozzle | 13: Rotameter |
| – | 7: Precipitator | 14: Solvent trap |






| Exp. No. | Operating Condition | Result | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | P (bar) | Conc. (%) | FCO2 (L/min) | Fsoln (mL/min) | N (μm) | Yield (%) | Crystal Form | Mean size (μm) | Crystal Habit | |
| Ori | --- | --- | --- | --- | --- | --- | --- | I | 6.10 | Irregular |
| 1 (a) | 35 | 100 | 90 | 4 | 0.5 | 100 | 91.8 | II | 88.9 | Needle-like/Nanocluster |
| 2 | 35 | 80 | 90 | 4 | 0.5 | 100 | 97.4 | II | 53.6 | Needle-like/Nanocluster |
| 3 | 35 | 120 | 90 | 4 | 0.5 | 100 | 92.9 | II | 71.4 | Needle-like |
| 4 | 35 | 100 | 30 | 4 | 0.5 | 100 | 87.4 | II | 60.3 | Needle-like/Nanocluster |
| 5 | 35 | 100 | 60 | 4 | 0.5 | 100 | 90.1 | II | 40.4 | Needle-like/Nanocluster |
| 6 | 35 | 100 | 90 | 2 | 0.5 | 100 | 91.2 | II | 79.2 | Needle-like/Nanocluster |
| 7 | 35 | 100 | 90 | 4 | 1.0 | 100 | 93.7 | II | 36.6 | Needle-like |
| 8 | 35 | 100 | 90 | 4 | 0.5 | 200 | 94.6 | II | 53.8 | Needle-like/Nanocluster |
| 9 (a) | 55 | 100 | 90 | 4 | 0.5 | 100 | 91.0 | I + II | 1.03 | Spherical |
| 10 | 55 | 80 | 90 | 4 | 0.5 | 100 | 89.1 | II | 4.32 | Rod-like |
| 11 | 55 | 120 | 90 | 4 | 0.5 | 100 | 91.4 | I + II | 2.23 | Spherical/irregular |
| 12 | 55 | 100 | 30 | 4 | 0.5 | 100 | 80.0 | I + II | 1.00 | Spherical |
| 13 | 55 | 100 | 60 | 4 | 0.5 | 100 | 83.7 | I + II | 0.91 | Spherical |
| 14 | 55 | 100 | 90 | 2 | 0.5 | 100 | 94.4 | I + II | 2.80 | Spherical/irregular |
| 15 | 55 | 100 | 90 | 4 | 1.0 | 100 | 93.3 | I + II | 1.37 | Spherical/irregular |
| 16 | 55 | 100 | 90 | 4 | 2.0 | 100 | 92.7 | II | 32.1 | Needle-like |
| 17 | 55 | 100 | 90 | 4 | 0.5 | 50 | 86.0 | I + II | 0.95 | Spherical |
| 18 | 55 | 100 | 90 | 4 | 0.5 | 200 | 81.9 | I + II | 1.17 | Spherical |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-Y.; Su, C.-S. Recrystallization and Production of Spherical Submicron Particles of Sulfasalazine Using a Supercritical Antisolvent Process. Crystals 2018, 8, 295. https://doi.org/10.3390/cryst8070295
Wu W-Y, Su C-S. Recrystallization and Production of Spherical Submicron Particles of Sulfasalazine Using a Supercritical Antisolvent Process. Crystals. 2018; 8(7):295. https://doi.org/10.3390/cryst8070295
Chicago/Turabian StyleWu, Wei-Yi, and Chie-Shaan Su. 2018. "Recrystallization and Production of Spherical Submicron Particles of Sulfasalazine Using a Supercritical Antisolvent Process" Crystals 8, no. 7: 295. https://doi.org/10.3390/cryst8070295
APA StyleWu, W. -Y., & Su, C. -S. (2018). Recrystallization and Production of Spherical Submicron Particles of Sulfasalazine Using a Supercritical Antisolvent Process. Crystals, 8(7), 295. https://doi.org/10.3390/cryst8070295