Bis(triphenylphosphine)iminium Salts of Dioxothiadiazole Radical Anions: Preparation, Crystal Structures, and Magnetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses
2.1.1. 3-Bromo-1,10-phenantroline-5,6-dione (Br-phendione) and 3,8-Dibromo-1,10-phenantroline-5,6-dione (diBr-phendione)
2.1.2. 5-Bromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-Dioxide (BrL)
2.1.3. 5,10-Dibromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide (diBrL)
2.1.4. Bis(triphenylphosphine)iminium [1,2,5]thiadiazole[3,4-f][1,10]phenanthroline 1,1-Dioxide H2O/(CH3)2CO Solvate (PPN(L))
2.1.5. Bis(triphenylphosphine)iminium [1,2,5]thiadiazole[3,4-f][4,7]phenanthroline 1,1-Dioxide (PPN(4,7-L))
2.1.6. Bis(triphenylphosphine)iminium 5-Bromo-[1,2,5]thiadiazole[3,4-f][1,10]phenanthroline 1,1-dioxide THF Solvate (PPN(BrL))
2.1.7. Bis(triphenylphosphine)iminium 5,10-Dibromo-[1,2,5]thiadiazole[3,4-f][1,10]phenanthroline 1,1-Dioxide (PPN(diBrL))
2.2. Other Physical Measurements
2.2.1. Magnetic Measurements
2.2.2. X-ray Diffraction Data Collection/Refinement
2.2.3. Calculation Details
3. Results
3.1. Syntheses
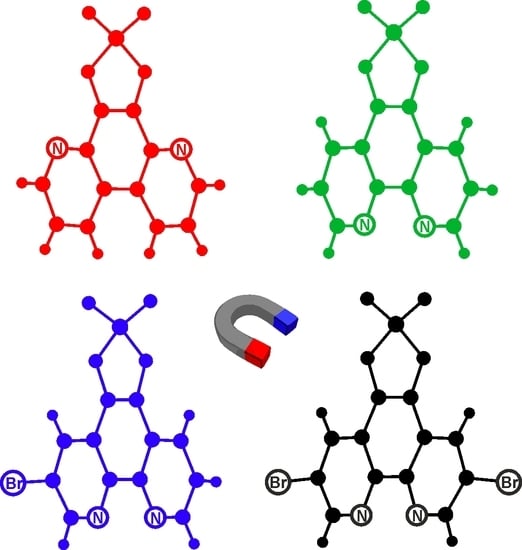
3.2. BrL and diBrL—Crystal Structures and DFT Calculations
3.3. BrL and diBrL—Cyclic Voltammetry
3.4. PPN Radical Salts—Crystal Structures
3.5. PPN+ Radical Salts—Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, J.S. Organic Magnets—A History. Adv. Mater. 2002, 14, 1105–1110. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Sieklucka, B.; Pinkowicz, D. Molecular Magnetic Materials: Concepts and Applications; Sieklucka, B., Pinkowicz, D., Eds.; WILEY-VCH: Weinheim, Germany, 2016; ISBN 352769420X. [Google Scholar]
- Ouahab, L. Multifunctional Molecular Materials; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; ISBN 9789814364294. [Google Scholar]
- Tezgerevska, T.; Alley, K.G.; Boskovic, C. Valence tautomerism in metal complexes: Stimulated and reversible intramolecular electron transfer between metal centers and organic ligands. Coord. Chem. Rev. 2014, 268, 23–40. [Google Scholar] [CrossRef]
- Himmel, H.-J. Valence tautomerism in copper coordination chemistry. Inorg. Chim. Acta 2017. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2014, 289–290, 149–176. [Google Scholar] [CrossRef]
- Demir, S.; Gonzalez, M.I.; Darago, L.E.; Evans, W.J.; Long, J.R. Giant coercivity and high magnetic blocking temperatures for N23-radical-bridged dilanthanide complexes upon ligand dissociation /639/638/263/406/639/638/911 /639/638/298/920 article. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A toolbox of building blocks, linkers and crystallisation methods used to generate single-chain magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Coronado, E. Molecule-based ferromagnetic conductors: Strategy and design. C. R. Chim. 2008, 11, 1110–1116. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical Conductivity Modulation Coupled to a High-Spin—Low-Spin Conversion in the Molecular System [FeIII(qsal)2][Ni(dmit)2]3‚ CH3CN‚ H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef]
- Bowman, A.C.; Tondreau, A.M.; Lobkovsky, E.; Margulieux, G.W.; Chirik, P.J. Synthesis and Electronic Structure Diversity of Pyridine(diimine)iron Tetrazene Complexes. Inorg. Chem. 2018, 57, 9634–9643. [Google Scholar] [CrossRef]
- Le Gal, Y.; Roisnel, T.; Auban-Senzier, P.; Bellec, N.; Iñiguez, J.; Canadell, E.; Lorcy, D. Stable Metallic State of a Neutral Radical Single-Component Conductor at Ambient Pressure. J. Am. Chem. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Wei, P.; Blair, S.A.; Cui, D.; Johnson, M.K.; Schaefer, H.F.; Robinson, G.H. Stable Boron Dithiolene Radicals. Angew. Chem. Int. Ed. 2018, 2–6. [Google Scholar] [CrossRef]
- Wang, Y.; Hickox, H.P.; Xie, Y.; Wei, P.; Blair, S.A.; Johnson, M.K.; Schaefer, H.F.; Robinson, G.H. A Stable Anionic Dithiolene Radical. J. Am. Chem. Soc. 2017, 139, 6859–6862. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.R.; Negru, B.; Van Duyne, R.P.; Harris, T.D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Chevalier, K.; Graf, M.; Schmitz, M.; Kelm, H.; Grün, A.; Zimmer, M.; Gerhards, M.; van Wüllen, C.; Krüger, H.J.; et al. Spectroscopic, Structural, and Kinetic Investigation of the Ultrafast Spin Crossover in an Unusual Cobalt(II) Semiquinonate Radical Complex. Chem. Eur. J. 2017, 23, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, H.; Li, L. Unusual Ln-radical chains constructed from functionalized nitronyl nitroxides: Synthesis, structure and magnetic properties. Inorg. Chem. Commun. 2017, 76, 59–61. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Li, L.; Sutter, J.-P. Lanthanide-Nitronyl Nitroxide Chains Derived from Multidentate Nitronyl Nitroxides. Inorg. Chem. 2018, 57, 7507–7511. [Google Scholar] [CrossRef]
- Calzado, C.J.; Rodríguez-García, B.; Galán Mascarós, J.R.; Hernández, N.C. Electronic Structure and Magnetic Interactions in the Radical Salt [BEDT-TTF]2[CuCl4]. Inorg. Chem. 2018, 57, 7077–7089. [Google Scholar] [CrossRef]
- Ke, X.; Hong, Y.; Lynch, V.M.; Kim, D.; Sessler, J.L. Metal-Stabilized Quinoidal Dibenzo[ g, p ]chrysene-Fused Bis- dicarbacorrole System. J. Am. Chem. Soc. 2018, 140, 7579–7586. [Google Scholar] [CrossRef]
- M, M.J.; Yee, G.T.; Mclean, R.S.; Epstein, A.J.; Miller, J.S. A ROOM-TEMPERATURE MOLECULAR ORGANIC BASED MAGNET. Science 1991, 252, 1415–1417. [Google Scholar] [CrossRef]
- Pokhodnya, K.I.; Bonner, M.; Her, J.H.; Stephens, P.W.; Miller, J.S. Magnetic ordering (Tc= 90 K) observed for layered [FeII(TCNE.-)(NCMe)2]+[FeIIICl4]-(TCNE = tetracyanoethylene). J. Am. Chem. Soc. 2006, 128, 15592–15593. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Elliott, R.W.; Hudson, T.A.; Robson, R.; Sutton, A.L. X4TCNQ2-dianions: Versatile building blocks for supramolecular systems. CrystEngComm 2018, 20, 3131–3152. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of charge transfer in a series of Ru2 II,II/TCNQ two-dimensional networks by tuning the electron affinity of TCNQ units: A route to synergistic magnetic/conducting materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Novitchi, G.; Shova, S.; Lan, Y.; Wernsdorfer, W.; Train, C. Verdazyl Radical, a Building Block for a Six-Spin-Center 2p–3d–4f Single-Molecule Magnet. Inorg. Chem. 2016, 55, 12122–12125. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shuku, Y.; Matsushita, M.M.; Awaga, K. Thiadiazole dioxide-fused picene: Acceptor ability, anion radical formation, and n-type charge transport characteristics. Chem. Commun. 2014, 50, 4178–4180. [Google Scholar] [CrossRef] [PubMed]
- Mirífico, M.V.; Caram, J.A.; Gennaro, A.M.; Cobos, C.J.; Vasini, E.J. Radical anions containing the dioxidated 1,2,5-thiadiazole heterocycle. Part II. J. Phys. Org. Chem. 2011, 24, 1039–1044. [Google Scholar] [CrossRef]
- Keller, S.N.; Bromby, A.D.; Sutherland, T.C. Optical Effect of Varying Acceptors in Pyrene Donor–Acceptor–Donor Chromophores. Eur. J. Org. Chem. 2017, 2017, 3980–3985. [Google Scholar] [CrossRef]
- Schüttler, C.; Li-Böhmer, Z.; Harms, K.; Von Zezschwitz, P. Enantioselective synthesis of 3,4-disubstituted cis- and trans-1,2,5-thiadiazolidine-1,1-dioxides as precursors for chiral 1,2-diamines. Org. Lett. 2013, 15, 800–803. [Google Scholar] [CrossRef]
- Arroyo, N.R.; Rozas, M.F.; Vázquez, P.; Romanelli, G.P.; Mirífico, M.V. Solvent-Free Condensation Reactions to Synthesize Five-Membered Heterocycles Containing the Sulfamide Fragment. Synth 2016, 48, 1344–1352. [Google Scholar] [CrossRef]
- Linder, T.; Badiola, E.; Baumgartner, T.; Sutherland, T.C. Synthesis of π-extended thiadiazole (oxides) and their electronic properties. Org. Lett. 2010, 12, 4520–4523. [Google Scholar] [CrossRef]
- Arslan, N.B.; Ertürk, A.G.; Kazak, C.; Bekdemir, Y. 3-Amino-4-[4-(dimethylamino)phenyl]-4,5-dihydro-1,2,5-thiadiazole 1,1-dioxide. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1736. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Li, Z.; Pietrzyk, P.; Rams, M. New thiadiazole dioxide bridging ligand with a stable radical form for the construction of magnetic coordination Chains. Cryst. Growth Des. 2014, 14, 4878–4881. [Google Scholar] [CrossRef]
- Shuku, Y.; Suizu, R.; Awaga, K. Monovalent and mixed-valent potassium salts of Thiadiazolo phenanthroline 1, 1-Dioxide: A radical anion for multidimensional network structures. Inorg. Chem. 2011, 50, 11859–11861. [Google Scholar] [CrossRef] [PubMed]
- Shuku, Y.; Awaga, K. Transition metal complexes and radical anion salts of 1,10-Phenanthroline derivatives annulated with a 1,2,5-Tiadiazole and 1,2,5-Tiadiazole 1,1-Dioxide Moiety: Multidimensional crystal structures and various magnetic properties. Molecules 2014, 19, 609–640. [Google Scholar] [CrossRef] [PubMed]
- Shuku, Y.; Suizu, R.; Domingo, A.; Calzado, C.J.; Robert, V.; Awaga, K. Multidimensional network structures and versatile magnetic properties of intermolecular compounds of a radical-anion ligand, [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 1,1-dioxide. Inorg. Chem. 2013, 52, 9921–9930. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef]
- Paw, W.; Eisenberg, R. Synthesis, Characterization, and Spectroscopy of Dipyridocatecholate Complexes of Platinum. Inorg. Chem. 1997, 36, 2287–2293. [Google Scholar] [CrossRef]
- Zhao, J.F.; Chen, L.; Sun, P.J.; Hou, X.Y.; Zhao, X.H.; Li, W.J.; Xie, L.H.; Qian, Y.; Shi, N.E.; Lai, W.Y.; et al. One-pot synthesis of 2-bromo-4,5-diazafluoren-9-one via a tandem oxidation-bromination-rearrangement of phenanthroline and its hammer-shaped donor-acceptor organic semiconductors. Tetrahedron 2011, 67, 1977–1982. [Google Scholar] [CrossRef]
- Bain, G.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Bruker. TWINABS; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Bruker. APEX3; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Guzei, I.A. An idealized molecular geometry library for refinement of poorly behaved molecular fragments with constraints. J. Appl. Crystallogr. 2014, 47, 806–809. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian09 Program; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Southerland, H.; Wang, X.-Y.; Dunbar, K. Record Antiferromagnetic Coupling for a 3d/4d Cyanide-Bridged. Compd. J. Am. Chem. Soc. 2014, 136, 9922–9924. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Southerland, H.; Avendano, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pederesen, K.S.; Dreiser, J.; Clérac, R.; Nehrkorn, J.; et al. Cyanide Single-Molecule Magnets Exhibiting Solvent Dependent Reversible “On” and “Off” Exchange Bias Behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef]






| Compound | Reduction Wave No. | Eox/mV | Ered/mV | E1/2/mV | Δ/eV | Ref. |
|---|---|---|---|---|---|---|
| diBrL | 1 | −387 | −495 | −441 | 3.183 | this work |
| 2 | −1139 | −1279 | −1208 | |||
| BrL | 1 | −405 | −538 | −471 | 3.352 | this work |
| 2 | −1166 | −1324 | −1245 | |||
| L | 1 | −499 | 3.562 | [35] | ||
| 2 | −1320 | |||||
| 4,7-L | 1 | −520 | 3.583 | [34] | ||
| 2 | −1229 |
| Compound | S=O | S-N | C=N | C-C |
|---|---|---|---|---|
| Br-L | 1.423(4) 1.429(4) | 1.693(5) 1.695(4) | 1.284(7) 1.293(6) | 1.505(7) |
| diBr-L | 1.421(4) 1.426(4) | 1.693(4) 1.696(5) | 1.289(7) 1.284(7) | 1.514(7) |
| av. in neutral molecules | 1.425 | 1.694 | 1.288 | 1.510 |
| PPN(4,7-L•) | 1.439(2) 1.436(2) | 1.648(2) 1.657(2) | 1.333(2) 1.333(2) | 1.443(2) |
| PPN(Br-L•) | 1.437(3) 1.433(3) | 1.649(3) 1.660(3) | 1.333(5) 1.332(5) | 1.441(5) |
| PPN(1,10-L•) | 1.442(2) 1.448(2) | 1.664(2) 1.664(3) | 1.342(3) 1.340(3) | 1.443(4) |
| PPN(diBr-L•) Molecule B | 1.443(4) 1.443(4) | 1.646(5) 1.660(5) | 1.336(7) 1.333(8) | 1.452(8) |
| PPN(diBr-L•) Molecule A | 1.442(4) 1.444(4) | 1.656(5) 1.660(5) | 1.338(8) 1.333(8) | 1.435(8) |
| av. in radical anions | 1.441 | 1.656 | 1.335 | 1.443 |
| Compound | χT(T) @300K/cm3 K mol−1 | χT(T) @80K/cm3 K mol−1 | χT(T) @1.8K/cm3 K mol−1 | M(H) @7T/µB | J/cm−1 |
|---|---|---|---|---|---|
| PPN(4,7-L) | 0.374 | 0.377 | 0.075 | 0.76 | −2(1) |
| PPN(BrL) | 0.374 | 0.363 | 0.024 | 0.20 | −4(1) |
| PPN(L) | 0.367 | 0.352 | 0.006 | 0.07 | −5(1) |
| PPN(diBrL) | 0.313 * | 0.200 | 0.157 ** | 0.37 | −116(10) −0.6(5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakulski, P.; Arczyński, M.; Pinkowicz, D. Bis(triphenylphosphine)iminium Salts of Dioxothiadiazole Radical Anions: Preparation, Crystal Structures, and Magnetic Properties. Crystals 2019, 9, 30. https://doi.org/10.3390/cryst9010030
Pakulski P, Arczyński M, Pinkowicz D. Bis(triphenylphosphine)iminium Salts of Dioxothiadiazole Radical Anions: Preparation, Crystal Structures, and Magnetic Properties. Crystals. 2019; 9(1):30. https://doi.org/10.3390/cryst9010030
Chicago/Turabian StylePakulski, Paweł, Mirosław Arczyński, and Dawid Pinkowicz. 2019. "Bis(triphenylphosphine)iminium Salts of Dioxothiadiazole Radical Anions: Preparation, Crystal Structures, and Magnetic Properties" Crystals 9, no. 1: 30. https://doi.org/10.3390/cryst9010030
APA StylePakulski, P., Arczyński, M., & Pinkowicz, D. (2019). Bis(triphenylphosphine)iminium Salts of Dioxothiadiazole Radical Anions: Preparation, Crystal Structures, and Magnetic Properties. Crystals, 9(1), 30. https://doi.org/10.3390/cryst9010030