Reversed Crystal Growth
Abstract
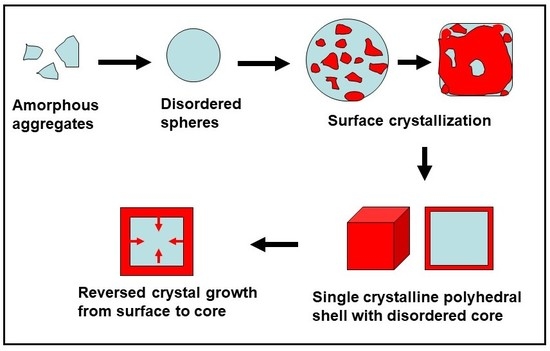
:1. Introduction
2. Reversed Crystal Growth
2.1. Zeolites
2.1.1. Zeolite Analcime
2.1.2. Zeolite A
2.1.3. Other Zeolites
2.2. Metal Organic Frameworks (MOF)
2.2.1. MOF-5
2.2.2. RHO-Zeolitic Imidazolate Framework (ZIF)
2.3. Metal Oxides
2.3.1. ZnO
2.3.2. Fe2O3
2.3.3. Perovskites ABO3
2.4. Biomimetic CaCO3
2.5. Metal Nanocrystals
2.6. Organic Crystals
3. Conclusions
Conflicts of Interest
References
- Bravais, A. Études Crystallographic; Gauthier-Villars: Paris, France, 1866. [Google Scholar]
- Friedel, M.G. Étudessurla loi de Bravais. Bull. Soc. Fr. Mineral Cristallogr. 1907, 30, 326–455. [Google Scholar]
- Donnay, D.; Harker, D. A new law of crystal morphology extending the law of Bravais. Am. Mineral. 1937, 22, 446–467. [Google Scholar]
- Hartman, P.; Perdok, W.G. On the relations between structure and morphology of crystals. II. Acta Crystallogr. 1955, 8, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Curie, P. Sur la formation des cristaux et sur les constants capillaires de leursdifférentes faces. Bull. Soc. Fr. Mineral Cristallogr. 1885, 8, 145–150. [Google Scholar]
- Wulff, G. Zur frage der geschwindigkeit des wachstums und der auflösung der kristallflächen. Z. Kristallogr. 1901, 34, 449–530. [Google Scholar]
- Prywer, J. On the crystal geometry influence on the growth of fast-growing surfaces. J. Phys. Chem. Solids 2002, 63, 493–501. [Google Scholar] [CrossRef]
- Prywer, J. Explanation of some peculiarities of crystal morphology deduced from the BFDH law. J. Cryst. Growth 2004, 270, 699–710. [Google Scholar] [CrossRef]
- Zhou, W.Z. Reversed crystal growth: Implications for crystal engineering. Adv. Mater. 2010, 22, 3086–3092. [Google Scholar] [CrossRef]
- Greer, H.F.; Yu, F.J.; Zhou, W.Z. Early stages of non-classic crystal growth. Sci. China Chem. 2011, 54, 1867–1876. [Google Scholar] [CrossRef]
- Chen, X.Y.; Qiao, M.H.; Xie, S.H.; Fan, K.N.; Zhou, W.Z.; He, H.Y. Self-construction of core-shell and hollow zeolite analcime icositetrahedra: A reversed crystal growth process via oriented aggregation of nanocrystallites and recrystallization from surface to core. J. Am. Chem. Soc. 2007, 129, 13305–13312. [Google Scholar] [CrossRef]
- Ueda, S.; Koizumi, M. Crystallization of analcime solid solutions from aqueous solutions. Am. Mineral. 1979, 64, 172–179. [Google Scholar]
- Wang, Y.; Li, X.G.; Xue, Z.Y.; Dai, L.S.; Xie, S.H.; Li, Q.Z. Preparation of zeolite ANA crystal from zeolite Y by in situ solid phase iso-structure transformation. J. Phys. Chem. B 2010, 114, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Samadi-Maybodi, A.; Pourali, S.M. Microwave-assisted hydrothermal green synthesis of analcime icositetrahedra: Insight into intermediates formed in the reversed crystal growth process. Eur. J. Inorg. Chem. 2014, 2014, 1204–1210. [Google Scholar] [CrossRef]
- Yao, J.F.; Li, D.; Zhang, X.Y.; Kong, C.H.; Yue, W.B.; Zhou, W.Z.; Wang, H.T. Cubes of zeolite A with an amorphous core. Angew. Chem. Int. Ed. 2008, 47, 8397–8399. [Google Scholar] [CrossRef] [PubMed]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W.Z. Early stage reversed crystal growth of zeolite A and its phase transformation to sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Singh, R.; Webley, P.A. Formation of LTA zeolite crystals with multi-hollow polycrystalline core–shell structure via aggregation–recrystallization route in presence of emulsion droplets. Microp. Mesop. Mater. 2012, 160, 75–84. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Zeng, C.F.; Zhang, L.X. Hydrothermal synthesis of pencil-like SAPO-5 and observation of its reversed crystal-growth process. Chem. Eur. J. 2013, 19, 365–371. [Google Scholar] [CrossRef]
- Gong, J.; Tong, F.; Ji, X.B.; Zeng, C.F.; Wang, C.Q.; Lv, Y.N.; Zhang, L.X. Hollow SAPO-34 cubes with hierarchically organized internal structure. Cryst. Growth Des. 2014, 14, 3857–3863. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Y.-X.; Pan, Y.M.; Mi, J.-X. Single-crystal microtubes of a novel apatite-type compound, (Na2.5Bi2.5)(PO4)3(F,OH), with well-faceted hexagonal cross sections. CrystEngComm 2009, 11, 1863–1867. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Jiang, J.; He, M.; Wu, P. Synthesis of ZSM-5 zeolite hollow spheres with a core/shell structure. J. Mater. Chem. 2010, 20, 10193–10199. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, D.; Yao, J.; He, L.; Ho, J.; Kong, C.; Hills, A.J.; Wang, H. In situ crystallization of macroporous monoliths with hollow NaP zeolite structure. Chem. Mater. 2010, 22, 5271–5278. [Google Scholar] [CrossRef]
- Van Vleet, M.J.; Weng, T.T.; Li, X.Y.; Schmidt, J.R. In situ, time-resolved, and mechanistic studies of metal−organic framework nucleation and growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Hafizovic, J.; Bjørgen, M.; Olsbye, U.; Dietzel, P.D.C.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Lillerud, K.P. The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 2007, 129, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Greer, H.F.; Chiang, C.-Y.; Zhou, W.Z. Microstructural study of formation mechanism of metal-organic framework MOF-5. CrystEngComm 2014, 16, 1064–1070. [Google Scholar] [CrossRef]
- Greer, H.F.; Liu, Y.H.; Greenaway, A.; Wright, P.A.; Zhou, W.Z. Synthesis and formation mechanism of textured MOF-5. Cryst. Growth Des. 2016, 16, 2104–2111. [Google Scholar] [CrossRef]
- Self, K.; Telfer, M.; Greer, H.F.; Zhou, W.Z. Reversed crystal growth of zeolite imidazolate framework RHO-ZIF. Chem. Euro. J. 2015, 21, 19090–19095. [Google Scholar] [CrossRef] [PubMed]
- Kahr, J.; Mowat, J.P.S.; Slawin, A.M.Z.; Morris, R.E.; Fairen-Jimenez, D.; Wright, P.A. Synthetic control of framework zinc purinate crystallisation and properties of a large pore, decorated, mixed-linker RHO-type ZIF. Chem. Commun. 2012, 48, 6690–6692. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.G.; Peng, Y.W.; Gao, Y.J.; Qian, Y.H.; Ying, S.M.; Yuan, D.Q.; Horike, S.; Ogiwara, N.; Babarao, R.; Wang, Y.X.; et al. Direct synthesis of hierarchically porous metal−organic frameworks with high stability and strong Brønsted acidity: The decisive role of hafnium in efficient and selective fructose dehydration. Chem. Mater. 2016, 28, 2659–2667. [Google Scholar] [CrossRef]
- Self, K.; Zhou, H.J.; Greer, H.F.; Tian, Z.R.; Zhou, W.Z. Reversed crystal growth of ZnO microdisks. Chem. Commun. 2013, 49, 5411–5413. [Google Scholar] [CrossRef]
- Greer, H.F.; Zhou, W.Z.; Liu, M.H.; Tseng, Y.H.; Mou, C.Y. The origin of ZnO twin crystals in bio-inspired synthesis. CrystEngComm 2012, 14, 1247–1255. [Google Scholar] [CrossRef]
- Liu, M.H.; Tseng, Y.H.; Greer, H.F.; Zhou, W.Z.; Mou, C.Y. Dipole field guided orientated attachment of nanocrystals to twin-brush ZnO mesocrystals. Chem. Euro. J. 2012, 18, 16104–16113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, R.T.; Dong, Y.P.; Li, W.; Zhou, W.Z. Preparation of α-Fe2O3 hollow spheres, nanotubes, nanoplates and nanorings as high efficient Cr(VI) adsorbents. RSC Adv. 2016, 6, 82854–82861. [Google Scholar] [CrossRef]
- Chen, J.L.; Macfarlane, S.; Zhang, C.X.; Yu, K.; Zhou, W.Z. Chemistry of hydrolysis of FeCl3 in the presence of phosphate to form hematite nanotubes and nanorings. Cryst. Growth Des. 2017, 17, 5975–5983. [Google Scholar] [CrossRef]
- Yang, X.F.; Fu, J.X.; Jin, C.J.; Chen, J.; Liang, C.L.; Wu, M.M.; Zhou, W.Z. Formation mechanism of CaTiO3 hollow crystals with different microstructures. J. Am. Chem. Soc. 2010, 132, 14279–14287. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.Q.; Chen, Z.-G.; Zhuang, J.L.; Yang, X.F.; Wu, Q.L.; Jiang, X.P.; Liang, C.L.; Wu, M.M.; Zou, J. Correlation between multiple growth stages and photocatalysis of SrTiO3 nanocrystals. J. Phys. Chem. C 2015, 119, 3530–3537. [Google Scholar] [CrossRef]
- Zhan, H.Q.; Yang, X.F.; Wang, C.M.; Chen, J.; Wen, Y.P.; Liang, C.L.; Greer, H.F.; Wu, M.M.; Zhou, W.Z. Multiple nucleation and crystal growth of barium titanate. Cryst. Growth Des. 2012, 12, 1247–1253. [Google Scholar] [CrossRef]
- Gao, J.B.; Shi, H.Y.; Yang, J.; Li, T.; Zhang, R.; Chen, D.L. Influencing factor investigation on dynamic hydrothermal growth of gapped hollow BaTiO3 nanospheres. Nanoscale Res. Lett. 2015, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.L.; Andrés, J.; Mastelaro, V.R.; Varela, J.A.; Longo, E. On the reversed crystal growth of BaZrO3 decaoctahedron: Shape evolution and mechanism. CrystEngComm 2011, 13, 5818–5824. [Google Scholar] [CrossRef]
- Ye, T.; Dong, Z.H.; Zhao, Y.N.; Yu, J.G.; Wang, F.Q.; Guo, S.K.; Zou, Y.C. Controllable fabrication of perovskite SrZrO3 hollow cuboidal nanoshells. CrystEngComm 2011, 13, 3842–3847. [Google Scholar] [CrossRef]
- Liu, S.J.; Gong, J.Y.; Hu, B.; Yu, S.H. Mesocrystals of rutile TiO2: Mesoscale transformation, crystallization, and growth by a biologic molecules-assisted hydrothermal process. Cryst. Growth Des. 2009, 9, 203–209. [Google Scholar] [CrossRef]
- Wu, S.T.; Chiang, C.-Y.; Zhou, W.Z. Formation mechanism of CaCO3 spherulites in myostracum layer of limpet shells. Crystals 2017, 7, 319. [Google Scholar] [CrossRef]
- Greer, H.F.; Liu, M.-H.; Mou, C.-Y.; Zhou, W.Z. Dipole field driven morphology evolution in biomimetic vaterite. CrystEngComm 2016, 18, 1585–1599. [Google Scholar] [CrossRef] [Green Version]
- Greer, H.F.; Zhou, W.Z.; Guo, L. Reversed crystal growth of calcite in naturally occurring travertine crust. Crystals 2017, 7, 36. [Google Scholar] [CrossRef]
- Ritchie, A.; Watson, M.; Turnbull, R.; Lu, Z.; Telfer, M.; Gano, J.; Self, K.; Greer, H.F.; Zhou, W.Z. Reversed crystal growth of rhombohedral calcite in the presence of chitosan and gum Arabic. CrystEngComm 2013, 15, 10266–10271. [Google Scholar] [CrossRef]
- Xie, S.H.; Zhou, W.Z.; Zhu, Y.Q. Formation mechanism of Mg2SiO4 fishbone-like fractal nanostructures. J. Phys. Chem. B 2004, 108, 11561–11566. [Google Scholar] [CrossRef]
- Yu, F.J.; Zhou, W.Z. Alloying and dealloying of CuPt bimetallic nanocrystals. Prog. Nat. Sci. Mater. Int. 2013, 23, 331–337. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Bučar, D.-K.; Baltrusaitis, J.; MacGillivray, L.R. Organic nanocrystals of the resorcinarene hexamer via sonochemistry: Evidence of reversed crystal growth involving hollow morphologies. J. Am. Chem. Soc. 2012, 134, 6900–6903. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.C.; Yang, P.P.; He, F.; Gai, S.L.; Yang, G.X.; Lin, J. Hollow structured Y2O3:Yb/Er−CuxS nanospheres with controllable size for simultaneous chemo/photothermal therapy and bioimaging. Chem. Mater. 2015, 27, 483–496. [Google Scholar] [CrossRef]
- Vasylkiv, O.; Bezdorozhev, O.; Sakka, Y. Synthesis of iron oxide nanoparticles with different morphologies by precipitation method with and without chitosan addition. J. Ceramic Soc. Jpn. 2016, 124, 489–494. [Google Scholar] [CrossRef] [Green Version]






© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W. Reversed Crystal Growth. Crystals 2019, 9, 7. https://doi.org/10.3390/cryst9010007
Zhou W. Reversed Crystal Growth. Crystals. 2019; 9(1):7. https://doi.org/10.3390/cryst9010007
Chicago/Turabian StyleZhou, Wuzong. 2019. "Reversed Crystal Growth" Crystals 9, no. 1: 7. https://doi.org/10.3390/cryst9010007
APA StyleZhou, W. (2019). Reversed Crystal Growth. Crystals, 9(1), 7. https://doi.org/10.3390/cryst9010007