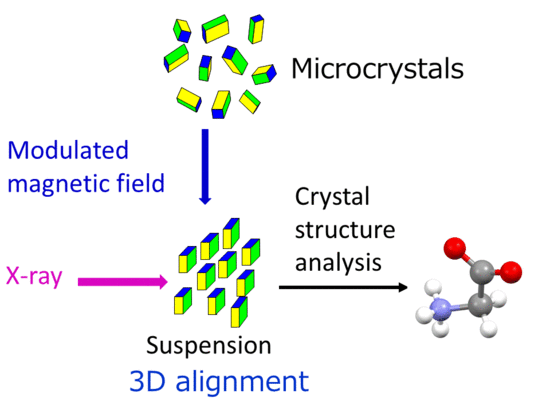
X-ray Single-Crystal Structural Analysis of a Magnetically Oriented Monoclinic Microcrystal Suspension of α-Glycine
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Kapustin, E.A.; Yin, S.X.; Liang, L.; Zhou, Z.; Niu, J.; Wang, X. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 2018, 361, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunaciu, A.A.; UdriŞTioiu, E.G.; Aboul-Enein, H.Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Margiolaki, I.; Wright, J.P.; Fitch, A.N.; Fox, G.C.; Labrador, A.; Von Dreele, R.B.; Miura, K.; Gozzo, F.; Schiltz, M.; Besnard, C.; et al. Powder diffraction studies on proteins: An overview of data collection approaches. Zeitschrift für Kristallographie Suppl. 2007, 26, 1–13. [Google Scholar] [CrossRef]
- Margiolakia, I.; Wrighta, J.P. Powder crystallography on macromolecules. Acta Crystallogr. Sect. A 2008, 64, 169–180. [Google Scholar] [CrossRef]
- Kimura, F.; Horii, S.; Arimoto, I.; Doi, T.; Yoshimura, M.; Wada, M.; Kimura, T. Determination of the anisotropic rotational diffusion constant of microcrystals dispersed in liquid medium. J. Phys. Chem. A 2018, 122, 9123–9127. [Google Scholar] [CrossRef]
- Kimura, F.; Horii, S.; Arimoto, I.; Notsu, D.; Doi, T.; Wada, M.; Kimura, T. X-ray diffraction study on the orientation dynamics of biaxial microcrystals under static and rotating magnetic fields. CrystEngComm 2019, 21, 4221–4226. [Google Scholar] [CrossRef]
- Tsuboi, C.; Aburaya, K.; Kimura, F.; Maeyama, M.; Kimura, T. Single-crystal structure determination from microcrystalline powders (~5 μm) by an orientation attachment mountable on an in-house X-ray diffractometer. CrystEngComm 2016, 18, 2404–2407. [Google Scholar] [CrossRef]
- Tsuboi, C.; Kimura, F.; Tanaka, T.; Kimura, T. Single-Crystal X-ray diffraction analysis of microcrystalline powders using magnetically oriented microcrystal suspensions. Cryst. Growth Des. 2016, 16, 2810–2813. [Google Scholar] [CrossRef]
- Tsuboi, C.; Tsukui, S.; Kimura, F.; Kimura, T.; Hasegawa, K.; Baba, S.; Mizuno, N. X-ray diffraction from magnetically oriented microcrystal suspensions detected by a shutterless continuous rotation method. J. Appl. Crystallogr. 2016, 49, 2100–2105. [Google Scholar] [CrossRef]
- Kimura, F.; Kimura, T.; Oshima, W.; Maeyama, M.; Aburaya, K. X-ray diffraction study of a pseudo single crystal prepared from a crystal belonging to point group 2. J. Appl. Crystallogr. 2010, 43, 151–153. [Google Scholar] [CrossRef]
- Tsukui, S.; Kimura, F.; Kusaka, K.; Baba, S.; Mizuno, N.; Kimura, T. Neutron and X-ray single-crystal diffraction from protein microcrystals via magnetically oriented microcrystal arrays in gels. Acta Crystallogr. Sect. D 2016, 72, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Staines, M. A Method of Biaxially Aligning Crystalline Material. PCT Pub. No. WO97/15067. 24 April 1997. [Google Scholar]
- Genoud, J.-Y.; Staines, M.; Mawdsley, A.; Manojlovic, V.; Quinton, W. Biaxially textured YBCO coated tape prepared using dynamic magnetic grain alignment. Supercond. Sci. Technol. 1999, 12, 663–671. [Google Scholar] [CrossRef]
- Kimura, T.; Chang, C.; Kimura, F.; Maeyama, M. The pseudo-single-crystal method: A third approach to crystal structure determination. J. Appl. Crystallogr. 2009, 42, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Oshima, W.; Matsumoto, H.; Uekusa, H.; Aburaya, K.; Maeyama, M.; Kimura, T. Single crystal structure analysis via magnetically oriented microcrystal arrays. CrystEngComm 2014, 16, 6630–6634. [Google Scholar] [CrossRef]
- Teranishi, S.; Kusumi, R.; Kimura, F.; Kimura, T.; Aburaya, K.; Maeyama, M. Biaxial magnetic orientation of zinc citrate as nucleating agent of poly (L-lactic acid). Chem. Lett. 2017, 46, 830–832. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Sato, Y. Method for Producing Glycine. JP 4557280. 23 June 2005. [Google Scholar]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. SHELXT Version 2014/5. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Crystal Structure Ver.4.3: Crystal Structure Analysis Package 2000–2018; Rigaku Corporation: Tokyo, Japan.
- Sheldrick, G.M. A short history of SHELX. SHELXL Version 2017/1. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yasuda, H.; Nagira, T.; Yoshiya, M. Three-dimensional alignment of FeSi2 with orthorhombic symmetry by an anisotropic magnetic field. J. Phys. Conf. Ser. 2009, 165, 12021. [Google Scholar] [CrossRef]
- Fukushima, T.; Horii, S.; Uchikoshi, T.; Ogino, H.; Ishihara, A.; Suzuki, T.S.; Sakka, Y.; Shimoyama, J.-I.; Kishio, K. 3-Dimensional Grain Orientation of RE-Ba-Cu-O Superconductors Using a Modulated Oval Magnetic Field. IEEE Trans. Appl. Supercond. 2009, 19, 2961. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ozawa, S.; Yamamoto, I.; Kimura, T. Characterization of Three-Dimensional Magnetic Alignment for Magnetically Biaxial Particles. Jpn. J. Appl. Phys. 2013, 52, 13003. [Google Scholar] [CrossRef]
- Yamamoto, S.; Terai, T.; Fukuda, T.; Sato, K.; Kakeshita, T.; Horii, S.; Ito, M.; Yonemura, M. Magnetocrystalline anisotropy of cementite pseudo single crystal fabricated under a rotating magnetic field. J. Magn. Magn. Mater. 2018, 451, 1–4. [Google Scholar] [CrossRef]
- Kimura, F.; Kimura, T. Magnetically textured powders—An alternative to single-crystal and powder X-ray diffraction methods. CrystEngComm 2018, 20, 861–872. [Google Scholar] [CrossRef]
- Kimura, T. Magnetically Oriented Microcrystal Arrays and Suspensions: Application to Diffraction Methods and Solid-State NMR Spectroscopy. In Advances in Organic Crystal Chemistry; Tamura, R., Miyata, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 167–186. ISBN 978-4-431-55555-1. [Google Scholar]
- Langan, P.; Mason, S.A.; Myles, D.; Schoenborn, B.P. Structural characterization of crystals of [alpha]-glycine during anomalous electrical behavior. Acta Crystallogr. Sect. B 2002, 58, 728–733. [Google Scholar] [CrossRef]







| Sample | α-Glycine MOMS | α-Glycine SC [25] |
|---|---|---|
| Crystal system | Monoclinic | monoclinic |
| Space group | P21/n | P21/n |
| Temperature (K) | 296 | 288 |
| a (Å) | 5.0833(10) | 5.0993(3) |
| b (Å) | 11.902(3) | 11.9416(6) |
| c (Å) | 5.4399(9) | 5.4608(3) |
| V (Å3) | 305.87(12) | 308.78(3) |
| Z | 4 | 4 |
| β (°) | 111.67(2) | 111.784(2) |
| 2θmax | 50.0 (Mo Kα) | 90.0 (neutrons) |
| Reflection | 412 | 1540 |
| Completeness | 0.770 | |
| Rint | 0.0939 | - |
| R1 [F2> 2σ(F2)] | 0.0595 | 0.0371 |
| wR2 [all data] | 0.1675 | 0.0777 |
| GOF | 1.241 | 1.204 |
| CCDC No. | 1957082 | 849662 Glycin96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Tsuboi, C.; Aburaya, K.; Kimura, F.; Maeyama, M.; Kimura, T. X-ray Single-Crystal Structural Analysis of a Magnetically Oriented Monoclinic Microcrystal Suspension of α-Glycine. Crystals 2019, 9, 561. https://doi.org/10.3390/cryst9110561
Tanaka T, Tsuboi C, Aburaya K, Kimura F, Maeyama M, Kimura T. X-ray Single-Crystal Structural Analysis of a Magnetically Oriented Monoclinic Microcrystal Suspension of α-Glycine. Crystals. 2019; 9(11):561. https://doi.org/10.3390/cryst9110561
Chicago/Turabian StyleTanaka, Tatsuya, Chiaki Tsuboi, Kazuaki Aburaya, Fumiko Kimura, Masataka Maeyama, and Tsunehisa Kimura. 2019. "X-ray Single-Crystal Structural Analysis of a Magnetically Oriented Monoclinic Microcrystal Suspension of α-Glycine" Crystals 9, no. 11: 561. https://doi.org/10.3390/cryst9110561
APA StyleTanaka, T., Tsuboi, C., Aburaya, K., Kimura, F., Maeyama, M., & Kimura, T. (2019). X-ray Single-Crystal Structural Analysis of a Magnetically Oriented Monoclinic Microcrystal Suspension of α-Glycine. Crystals, 9(11), 561. https://doi.org/10.3390/cryst9110561