New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. X-ray Structure Determination
2.3. Computational Details
3. Results and Discussion
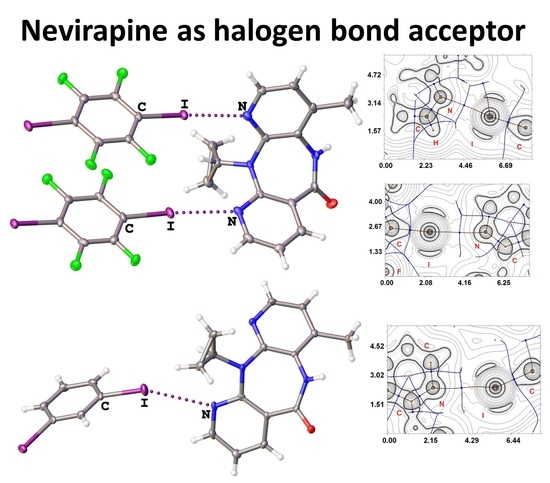
3.1. Halogen Bonding in NVP·1,4-FIB and NVP·1,3-DIB
3.2. Hydrogen Bonding in the NVP·1,4-FIB and NVP·1,3-DIB Co-Crystals
3.3. Theoretical Study of Different Noncovalent Interactions in NVP·1,4-FIB and NVP·1,3-DIB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jie, L.; Sohrab, R. Polymorphism and Crystallization of Active Pharmaceutical Ingredients (APIs). Curr. Med. Chem. 2009, 16, 884–905. [Google Scholar] [CrossRef]
- Yin, S.X.; Grosso, J.A. Selecting and Controlling API Crystal Form for Pharmaceutical Development—Strategies and Processes. Curr. Opin. Drug Discov. Dev. 2008, 11, 771–777. [Google Scholar]
- Karpinski, P.H. Polymorphism of active pharmaceutical ingredients. Chem. Eng. Technol. 2006, 29, 233–237. [Google Scholar] [CrossRef]
- Mancera, R.L. Molecular modeling of hydration in drug design. Curr. Opin. Drug Discov. Dev. 2007, 10, 275–280. [Google Scholar]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Grant, D.J.W. Crystal structures of drugs: Advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef]
- Trask, A.V. An overview of pharmaceutical cocrystals as intellectual property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Zaworotko, M.J. Manifestations of noncovalent bonding in the solid state. 6. [H4(cyclam)]4+ (cyclam=1,4,8,11-tetraazacyclotetradecane) as a template for crystal engineering of network hydrogen-bonded solids. Can. J. Chem. 1995, 73, 414–424. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [Green Version]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical cocrystallization: Engineering a remedy for caffeine hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Leyssens, T.; Tumanova, N.; Robeyns, K.; Candoni, N.; Veesler, S. Solution cocrystallization, an effective tool to explore the variety of cocrystal systems: Caffeine/dicarboxylic acid cocrystals. CrystEngComm 2014, 16, 9603–9611. [Google Scholar] [CrossRef]
- Braga, D.; Palladino, G.; Polito, M.; Rubini, K.; Grepioni, F.; Chierotti, M.R.; Gobetto, R. Three Polymorphic Forms of the Co-Crystal 4,4′-Bipyridine/Pimelic Acid and their Structural, Thermal, and Spectroscopic Characterization. Chem. Eur. J. 2008, 14, 10149–10159. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Mavračić, J.; Cinčić, D.; Kaitner, B. Halogen bonding of N-bromosuccinimide by grinding. CrystEngComm 2016, 18, 3343–3346. [Google Scholar] [CrossRef] [Green Version]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Choquesillo-Lazarte, D.; Nemec, V.; Cinčić, D. Halogen bonded cocrystals of active pharmaceutical ingredients: Pyrazinamide, lidocaine and pentoxifylline in combination with haloperfluorinated compounds. CrystEngComm 2017, 19, 5293–5299. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef] [Green Version]
- Merkens, C.; Pan, F.F.; Englert, U. 3-(4-Pyridyl)-2,4-pentanedione—A bridge between coordinative, halogen, and hydrogen bonds. CrystEngComm 2013, 15, 8153–8158. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Structural Equivalence of Br and I Halogen Bonds: A Route to Isostructural Materials with Controllable Properties. Chem. Mat. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Tan, D.; Barrett, C.J.; Friščić, T. Fluorinated azobenzenes with highly strained geometries for halogen bond-driven self-assembly in the solid state. CrystEngComm 2015, 17, 73–80. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T. Synthesis of an extended halogen-bonded metal-organic structure in a one-pot mechanochemical reaction that combines covalent bonding, coordination chemistry and supramolecular synthesis. CrystEngComm 2014, 16, 10169–10172. [Google Scholar] [CrossRef]
- Troff, R.W.; Mäkelä, T.; Topić, F.; Valkonen, A.; Raatikainen, K.; Rissanen, K. Alternative Motifs for Halogen Bonding. Eur. J. Org. Chem. 2013, 1617–1637. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. [Google Scholar] [CrossRef]
- Carletta, A.; Spinelli, F.; d’Agostino, S.; Ventura, B.; Chierotti, M.R.; Gobetto, R.; Wouters, J.; Grepioni, F. Halogen-Bond Effects on the Thermo- and Photochromic Behaviour of Anil-Based Molecular Co-crystals. Chem. Eur. J. 2017, 23, 5317–5329. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinska, M.; Fokt, I.; Priebe, W.; Wozniak, K. Bromine Atom Interactions in Biologically Active Acrylamide Derivatives. Cryst. Growth Des. 2015, 15, 2632–2642. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Welideniya, D.; Desper, J.; Moore, C. Halogen-bond driven co-crystallization of potential anti-cancer compounds: A structural study. CrystEngComm 2014, 16, 10203–10209. [Google Scholar] [CrossRef]
- Baldrighi, M.; Bartesaghi, D.; Cavallo, G.; Chierotti, M.R.; Gobetto, R.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Polymorphs and co-crystals of haloprogin: An antifungal agent. CrystEngComm 2014, 16, 5897–5904. [Google Scholar] [CrossRef]
- Baldrighi, M.; Cavallo, G.; Chierotti, M.R.; Gobetto, R.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen Bonding and Pharmaceutical Cocrystals: The Case of a Widely Used Preservative. Mol. Pharm. 2013, 10, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Kirina, Y.V.; Kukushkin, V.Y. Halogen bonding between metal centers and halocarbons. Chem. Commun. 2016, 52, 5565–5568. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, D.M.; Novikov, A.S.; Starova, G.L.; Haukka, M.; Kukushkin, V.Y. A family of heterotetrameric clusters of chloride species and halomethanes held by two halogen and two hydrogen bonds. CrystEngComm 2016, 18, 5278–5286. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H2C(X)–X···X– (X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Avdontceva, M.S.; Kukushkin, V.Y. Diiodomethane as a halogen bond donor toward metal-bound halides. CrystEngComm 2017, 19, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic-Nucleophilic Dualism of Nickel(II) toward Ni···I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via -Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef] [PubMed]
- Rozhkov, A.V.; Novikov, A.S.; Ivanov, D.M.; Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Structure-Directing Weak Interactions with 1,4-Diiodotetrafluorobenzene Convert One-Dimensional Arrays of [MII(acac)2] Species into Three-Dimensional Networks. Cryst. Growth Des. 2018, 18, 3626–3636. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent···Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef] [PubMed]
- Baykov, S.V.; Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Boyarskiy, V.P. Pt/Pd and I/Br Isostructural Exchange Provides Formation of C–I···Pd, C–Br···Pt, and C–Br···Pd Metal-Involving Halogen Bonding. Cryst. Growth Des. 2018, 18, 5973–5980. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Bikbaeva, Z.M.; Bokach, N.A.; Kukushkin, V.Y. Noncovalent Interactions Involving Iodofluorobenzenes: The Interplay of Halogen Bonding and Weak lp(O)···π-Holearene Interactions. Cryst. Crowth Des. 2018, 18, 7641–7654. [Google Scholar] [CrossRef]
- Sapegin, A.; Krasavin, M. Efficient Suzuki-Miyaura mono-arylation of symmetrical diiodo(hetero) arenes. Tetrahedron Lett. 2018, 59, 1948–1951. [Google Scholar] [CrossRef]
- Bartlett, J.G. The DHHS adult ART guidelines are revised. Hopkins HIV Rep. 2005, 17, 6–7. [Google Scholar] [PubMed]
- Bardsley-Elliot, A.; Perry, C.M. Nevirapine: A review of its use in the prevention and treatment of paediatric HIV infection. Paediatr. Drugs 2000, 2, 373–407. [Google Scholar] [CrossRef]
- Gazzard, B. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2005). HIV Med. 2005, 6, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Landriscina, M.; Bagala, C.; Piscazzi, A.; Schinzari, G.; Quirino, M.; Fabiano, A.; Bianchetti, S.; Cassano, A.; Sica, G.; Barone, C. Nevirapine Restores Androgen Signaling in Hormone-Refractory Human Prostate Carcinoma Cells Both In Vitro and In Vivo. Prostate 2009, 69, 744–754. [Google Scholar] [CrossRef]
- Landriscina, M.; Spadafora, C.; Cignarelli, M.; Barone, C. Anti-tumor activity of non-nucleosidic reverse transcriptase inhibitors. Curr. Pharm. Des. 2007, 13, 737–747. [Google Scholar] [CrossRef]
- Stefanidis, K.; Loutradis, D.; Vassiliou, L.-V.; Anastasiadou, V.; Kiapekou, E.; Nikas, V.; Patris, G.; Vlachos, G.; Rodolakis, A.; Antsaklis, A. Nevirapine induces growth arrest and premature senescence in human cervical carcinoma cells. Gynecol. Oncol. 2008, 111, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mui, P.W.; Jacober, S.P.; Hargrave, K.D.; Adams, J. Crystal structure of nevirapine, a nonnucleoside inhibitor of HIV-1 reverse-transcriptase, and computational alignment with a structurally diverse inhibitor. J. Med. Chem. 1992, 35, 201–202. [Google Scholar] [CrossRef]
- Da Silva, C.C.P.; Cuffini, S.L.; Faudone, S.N.; Ayala, A.P.; Ellena, J. Low-temperature study of a new nevirapine pseudopolymorph. Acta Crystallogr. Sect. E Struct Rep. Online 2008, 64, O292. [Google Scholar] [CrossRef] [PubMed]
- Stieger, N.; Liebenberg, W.; Wessels, J.C.; Samsodien, H.; Caira, M.R. Channel inclusion of primary alcohols in isostructural solvates of the antiretroviral nevirapine: An X-ray and thermal analysis study. Struct. Chem. 2010, 21, 771–777. [Google Scholar] [CrossRef]
- Caira, M.R.; Bourne, S.A.; Samsodien, H.; Engel, E.; Liebenberg, W.; Stieger, N.; Aucamp, M. Co-crystals of the antiretroviral nevirapine: Crystal structures, thermal analysis and dissolution behaviour. CrystEngComm 2012, 14, 2541–2551. [Google Scholar] [CrossRef]
- Stieger, N.; Caira, M.R.; Liebenberg, W.; Tiedt, L.R.; Wessels, J.C.; De Villiers, M.M. Influence of the Composition of Water/Ethanol Mixtures on the Solubility and Recrystallization of Nevirapine. Cryst. Growth Des. 2010, 10, 3859–3868. [Google Scholar] [CrossRef]
- Caira, M.R.; Stieger, N.; Liebenberg, W.; De Villieris, M.M.; Samsodien, H. Solvent inclusion by the anti-HIV drug nevirapine: X-ray structures and thermal decomposition of representative solvates. Cryst. Growth Des. 2008, 8, 17–23. [Google Scholar] [CrossRef]
- Pereira, B.G.; Fonte-Boa, F.D.; Resende, J.; Pinheiro, C.B.; Fernandes, N.G.; Yoshida, M.I.; Vianna-Soares, C.D. Pseudopolymorphs and intrinsic dissolution of nevirapine. Cryst. Growth Des. 2007, 7, 2016–2023. [Google Scholar] [CrossRef]
- Yang, X.T.; Yu, B.H.; Zhong, Z.; Guo, B.H.; Huang, Y.B. Nevirapine-polycaprolactone crystalline inclusion complex as a potential long-acting injectable solid form. Int. J. Pharm. 2018, 543, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kryukova, M.A.; Sapegin, A.V.; Novikov, A.S.; Krasavin, M.; Ivanov, D.M. Noncovalent interactions observed in nevirapinium pentaiodide hydrate which include the rare I4–I–···O=C halogen bonding. Z. Kristallogr. Cryst. Mater. 2018, in press. [Google Scholar] [CrossRef]
- Capucci, D.; Balestri, D.; Mazzeo, P.P.; Pelagatti, P.; Rubini, K.; Bacchi, A. Liquid Nicotine Tamed in Solid Forms by Cocrystallization. Cryst. Growth Des. 2017, 17, 4958–4964. [Google Scholar] [CrossRef]
- Sugiyama, H.; Uekusa, H. Relationship between crystal structures and photochromic properties of N-salicylideneaminopyridine derivatives. CrystEngComm 2018, 20, 2144–2151. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Agilent Technologies Ltd. CrysAlisPro; Version 1.171.136.120 (release 127-106-2012); Agilent Technologies Ltd.: Santa Clara, CA, USA, 2012. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Barros, C.L.; de Oliveira, P.J.P.; Jorge, F.E.; Canal Neto, A.; Campos, M. Gaussian basis set of double zeta quality for atoms Rb through Xe: Application in non-relativistic and relativistic calculations of atomic and molecular properties. Mol. Phys. 2010, 108, 1965–1972. [Google Scholar] [CrossRef]
- De Berrêdo, R.C.; Jorge, F.E. All-electron double zeta basis sets for platinum: Estimating scalar relativistic effects on platinum(II) anticancer drugs. J. Mol. Struct. THEOCHEM 2010, 961, 107–112. [Google Scholar] [CrossRef]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas–Kroll–Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef]
- Neto, A.C.; Jorge, F.E. All-electron double zeta basis sets for the most fifth-row atoms: Application in DFT spectroscopic constant calculations. Chem. Phys. Lett. 2013, 582, 158–162. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Roper, L.C.; Prasang, C.; Kozhevnikov, V.N.; Whitwood, A.C.; Karadakov, P.B.; Bruce, D.W. Experimental and Theoretical Study of Halogen-Bonded Complexes of DMAP with Di- and Triiodofluorobenzenes. A Complex with a Very Short N···I Halogen Bond. Cryst. Growth Des. 2010, 10, 3710–3720. [Google Scholar] [CrossRef]
- Eccles, K.S.; Morrison, R.E.; Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Investigating C=S···I Halogen Bonding for Cocrystallization with Primary Thioamides. Cryst. Growth Des. 2015, 15, 3442–3451. [Google Scholar] [CrossRef]
- Walsh, R.B.; Padgett, C.W.; Metrangolo, P.; Resnati, G.; Hanks, T.W.; Pennington, W.T. Crystal engineering through halogen bonding: Complexes of nitrogen heterocycles with organic iodides. Cryst. Growth Des. 2001, 1, 165–175. [Google Scholar] [CrossRef]
- Yan, D.P.; Delori, A.; Lloyd, G.O.; Friščić, T.; Day, G.M.; Jones, W.; Lu, J.; Wei, M.; Evans, D.G.; Duan, X. A Cocrystal Strategy to Tune the Luminescent Properties of Stilbene-Type Organic Solid-State Materials. Angew. Chem. Int. Ed. 2011, 50, 12483–12486. [Google Scholar] [CrossRef] [PubMed]
- Ravat, P.; SeethaLekshmi, S.; Biswas, S.N.; Nandy, P.; Varughese, S. Equivalence of Ethylene and Azo-Bridges in the Modular Design of Molecular Complexes: Role of Weak Interactions. Cryst. Growth Des. 2015, 15, 2389–2401. [Google Scholar] [CrossRef]
- Cerreia Vioglio, P.; Catalano, L.; Vasylyeva, V.; Nervi, C.; Chierotti, M.R.; Resnati, G.; Gobetto, R.; Metrangolo, P. Natural Abundance 15N and 13C Solid-State NMR Chemical Shifts: High Sensitivity Probes of the Halogen Bond Geometry. Chem. Eur. J. 2016, 22, 16817–16826. [Google Scholar] [CrossRef]
- Ji, B.M.; Wang, W.Z.; Deng, D.S.; Zhang, Y.; Cao, L.; Zhou, L.; Ruan, C.S.; Li, T.S. Structural competition between π···π interactions and halogen bonds: A crystallographic study. CrystEngComm 2013, 15, 769–774. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular hydrogen bond energies in crystals evaluated using electron density properties: DFT computations with periodic boundary conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.S.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.J.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem. Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Avdontceva, M.S.; Kukushkin, V.Y. Ligation-Enhanced π-Hole···π Interactions Involving Isocyanides: Effect of -Hole···Noncovalent Bonding on Conformational Stabilization of Acyclic Diaminocarbene Ligands. Inorg. Chem. 2018, 57, 6722–6733. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Bondarenko, M.A.; Abramov, P.A.; Novikov, A.S.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. Bromo- and Polybromoantimonates(V): Structural and Theoretical Studies of Hybrid Halogen-Rich Halometalate Frameworks. Chem. Eur. J. 2018, 24, 10165–10170. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Novikov, A.S.; Kinzhalov, M.A.; Boyarskiy, V.P.; Starova, G.L.; Ivanov, A.Y.; Kukushkin, V.Y. Halides Held by Bifurcated Chalcogen-Hydrogen Bonds. Effect of μ(S,N-H)-Cl-(S,Cl-N-H) Contacts on Dimerization of Cl(carbene)PdII Species. Inorg. Chem. 2018, 57, 3420–3433. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, S.J. Noncovalent interactions—QTAIM and NBO analysis. J. Mol. Model. 2013, 19, 4713–4721. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H···F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]



| Structure | C–I···N | d(I···N), Å | RIN 2 | ∠(C–I···N), ° | Eint 3 | Eint 4 |
|---|---|---|---|---|---|---|
| NVP·1,3-DIB | C1S–I1S···N1 | 3.231(3) | 0.92 | 156.09(11) | 2.5 | 2.4 |
| NVP·1,4-FIB | C1S–I1S···N1 | 2.988(4) | 0.85 | 174.33(10) | 4.4 | 3.8 |
| C2S–I2S···N4 | 2.973(4) | 0.84 | 175.88(13) | 4.4 | 4.0 | |
| Comparison1 | 3.53 | 1.00 | 180 |
| Contact | ρ(r) | ∇2ρ(r) | Hb | V(r) | G(r) | Eint 1 | Eint 2 | l | WI | DI |
|---|---|---|---|---|---|---|---|---|---|---|
| (NVP)4·(1,4-FIB)3 | ||||||||||
| I1S···N1 | 0.019 | 0.060 | 0.001 | −0.014 | 0.014 | 4.4 | 3.8 | 2.988 | 0.04 | 0.00 |
| I2S···N4 | 0.019 | 0.062 | 0.001 | −0.014 | 0.015 | 4.4 | 4.0 | 2.973 | 0.04 | 0.01 |
| H3···O1 | 0.024 | 0.097 | 0.003 | −0.017 | 0.021 | 5.3 | 5.7 | 1.980 | 0.02 | 0.00 |
| H9···O1 | 0.005 | 0.020 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 2.702 | 0.00 | 0.00 |
| H2···N4 | 0.008 | 0.030 | 0.001 | −0.004 | 0.006 | 1.3 | 1.6 | 2.601 | 0.00 | 0.00 |
| H15B···F3S | 0.005 | 0.026 | 0.001 | −0.004 | 0.005 | 1.3 | 1.3 | 2.597 | 0.00 | 0.00 |
| H12B···F4S | 0.006 | 0.032 | 0.002 | –0.005 | 0.006 | 1.6 | 1.6 | 2.461 | 0.00 | 0.00 |
| H10···F1S | 0.007 | 0.034 | 0.002 | −0.005 | 0.007 | 1.6 | 1.9 | 2.500 | 0.00 | 0.00 |
| H13···I1S | 0.006 | 0.026 | 0.002 | −0.003 | 0.005 | 0.9 | 1.3 | 3.145 | 0.00 | 0.00 |
| H13···I2S | 0.010 | 0.033 | 0.001 | −0.006 | 0.007 | 1.9 | 1.9 | 2.931 | 0.01 | 0.00 |
| C9···C11 | 0.007 | 0.022 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 3.237 | 0.00 | 0.00 |
| C2S···C2S | 0.006 | 0.019 | 0.001 | −0.002 | 0.004 | 0.6 | 1.1 | 3.364 | 0.00 | 0.00 |
| C2···C4S | 0.006 | 0.018 | 0.001 | −0.002 | 0.004 | 0.6 | 1.1 | 3.372 | 0.00 | 0.00 |
| (NVP)3·(1,3-DIB)3 | ||||||||||
| I1S···N1 | 0.012 | 0.043 | 0.001 | −0.008 | 0.009 | 2.5 | 2.4 | 3.231 | 0.02 | 0.00 |
| H3···O1 | 0.020 | 0.086 | 0.003 | −0.015 | 0.018 | 4.7 | 4.8 | 2.035 | 0.01 | 0.01 |
| H12A···O1 | 0.007 | 0.033 | 0.002 | −0.004 | 0.006 | 1.3 | 1.6 | 2.585 | 0.00 | 0.00 |
| H2···N4 | 0.007 | 0.031 | 0.002 | −0.004 | 0.006 | 1.3 | 1.6 | 2.666 | 0.00 | 0.00 |
| H6S···I2S | 0.005 | 0.020 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 3.172 | 0.00 | 0.00 |
| I2S···I2S | 0.014 | 0.043 | 0.001 | −0.010 | 0.010 | 3.1 | 2.7 | 3.562 | 0.04 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryukova, M.A.; Sapegin, A.V.; Novikov, A.S.; Krasavin, M.; Ivanov, D.M. New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors. Crystals 2019, 9, 71. https://doi.org/10.3390/cryst9020071
Kryukova MA, Sapegin AV, Novikov AS, Krasavin M, Ivanov DM. New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors. Crystals. 2019; 9(2):71. https://doi.org/10.3390/cryst9020071
Chicago/Turabian StyleKryukova, Mariya A., Alexander V. Sapegin, Alexander S. Novikov, Mikhail Krasavin, and Daniil M. Ivanov. 2019. "New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors" Crystals 9, no. 2: 71. https://doi.org/10.3390/cryst9020071
APA StyleKryukova, M. A., Sapegin, A. V., Novikov, A. S., Krasavin, M., & Ivanov, D. M. (2019). New Crystal Forms for Biologically Active Compounds. Part 1: Noncovalent Interactions in Adducts of Nevirapine with XB Donors. Crystals, 9(2), 71. https://doi.org/10.3390/cryst9020071