Synthesis, Structures and Co-Crystallizations of Perfluorophenyl Substituted β-Diketone and Triketone Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of H1 and H22
2.3. Co-Crystallizations of H1•H3 and H22•H24
2.4. Crystal Structure Determination
3. Results and Discussion
3.1. Enol-Type Structures of H1 and 2
3.2. UV-Vis Studies of the Perfluorinated Compounds
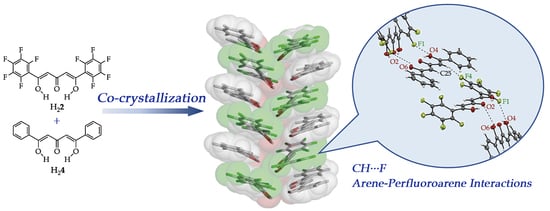
3.3. Co-Crystallization by Arene-Perfluoroarene Interactions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; IUCR, Ed.; Oxford University Press: Oxford, UK, 1999; pp. 202–224. [Google Scholar]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnatti, G. Halogen bonding based recognition processes: A world parallel to hydrogen bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef]
- Shimizu, K.; Ferreira da Silva, J. Halogen and hydrogen bonding interplay in the crystal packing of halometallocenes. Molecules 2018, 23, 2959. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kondo, M.; Kanaikea, M.; Masaoka, S. Arene–perfluoroarene interactions for crystal engineering of metal complexes: Controlled self-assembly of paddle-wheel dimers. CrystEngComm 2013, 15, 6122–6126. [Google Scholar] [CrossRef]
- Bouyahyi, M.; Roisnel, T.; Carpentier, J.-F. Aluminum complexes of fluorinated β-diketonate ligands: Syntheses, structures, intramolecular reduction, and use in ring-opening polymerization of lactide. Organometallics 2010, 29, 491–500. [Google Scholar] [CrossRef]
- Hernández-Trujillo, J.; Vela, A. Molecular quadrupole moments for the series of fluoro- and chlorobenzenes. J. Phys. Chem. 1996, 100, 6524–6530. [Google Scholar] [CrossRef]
- Doerksen, R.J.; Thakkar, A.J. Quadrupole and octopole moments of heteroaromatic rings. J. Phys. Chem. A 1999, 103, 10009–10014. [Google Scholar] [CrossRef]
- Williams, J.H. The molecular electric quadrupole moment and solid-state architecture. Acc. Chem. Res. 1993, 26, 593–598. [Google Scholar] [CrossRef]
- Patrick, C.R.; Prosser, G.S. A molecular complex of benzene and hexafluorobenzene. Nature 1960, 187, 1021. [Google Scholar] [CrossRef]
- Hori, A. The importance of π-interactions in crystal enigineering; Tiekink, E.R., Zukerman-Schpector, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 163–185. [Google Scholar]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic rings in chemical and biological recognition: Energetics and structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef]
- Coates, G.W.; Dunn, A.R.; Henling, L.M.; Dougherty, D.A.; Grubbs, R.H. Phenyl-perfluorophenyl stacking interactions: A new strategy for supermolecule construction. Angew. Chem. Int. Ed. Engl. 1997, 36, 248–251. [Google Scholar] [CrossRef]
- Kilbinger, A.F.M.; Grubbs, R.H. Arene-perfluoroarene interactions as physical crosslinks for hydrogel formation. Angew. Chem. Int. Ed. 2002, 41, 1563–1566. [Google Scholar] [CrossRef]
- Vangala, V.R.; Nangia, A.; Lynch, V.M. Interplay of phenyl-perfluorophenyl stacking, C-H···F, C-F···π and F···F interactions in some crystalline aromatic azines. Chem. Commun. 2002, 1304–1305. [Google Scholar] [CrossRef]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in crystal engineering—“The little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Gramlich, V.; Frauenrath, H. Alternating diacetylene copolymer utilizing perfluorophenyl-phenyl interactions. J. Am. Chem. Soc. 2006, 128, 5541–5547. [Google Scholar] [CrossRef]
- Dai, C.; Nguyen, P.; Marder, T.B.; Scott, A.J.; Clegg, W.; Viney, C. Control of single crystal structure and liquid crystal phase behaviour via arene-perfluoroarene interactions. Chem. Commun. 1999, 2493–2494. [Google Scholar] [CrossRef]
- Collings, J.C.; Roscoe, K.P.; Robins, E.G.; Batsanov, A.S.; Stimson, L.M.; Howard, J.A.K.; Clark, S.J.; Marder, T.B. Arene-perfluoroarene interactions in crystal engineering. 8: Structures of 1:1 complexes of hexafluorobenzene with fused-ring polyaromatic hydrocarbons. New J. Chem. 2002, 26, 1740–1746. [Google Scholar] [CrossRef]
- Fasina, T.M.; Collings, J.C.; Lydon, D.P.; Albesa-Jove, D.; Batsanov, A.S.; Howard, J.A.K.; Nguyen, P.; Bruce, M.; Scott, A.J.; Clegg, W.; et al. Synthesis, optical properties, crystal structures and phase behaviour of selectively fluorinated 1,4-bis(4′-pyridylethynyl)benzenes, 4-(phenylethynyl)pyridines and 9,10-bis(4′-pyridylethynyl)anthracene, and a Zn(NO3)2 coordination polymer. J. Mater. Chem. 2004, 14, 2395–2404. [Google Scholar] [CrossRef]
- Collings, J.C.; Batsanov, A.S.; Howard, J.A.K.; Dickie, D.A.; Clyburne, J.A.C.; Jenkins, H.A.; Marder, T.B. 1:1 Complexes of octafluoronaphthalene with trans-stilbene and trans-azobenzene. J. Fluor. Chem. 2005, 126, 515–519. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Collings, J.C.; Marder, T.B. Arene-perfluoroarene interactions in crystal engineering. XV. Ferrocene-decafluorobiphenyl (1/1). Acta Cryst. 2006, C62, m229–m231. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Haneline, M.R.; Tsunoda, M.; Gabbaï, F.P. π-Complexation of biphenyl, naphthalene, and triphenylene to trimeric perfluoro-ortho-phenylene mercury. Formation of extended binary stacks with unusual luminescent properties. J. Am. Chem. Soc. 2002, 124, 3737–3742. [Google Scholar] [CrossRef]
- Taylor, T.J.; Bakhmutov, V.I.; Gabbaï, F.P. Hydrocarbon uptake in the alkylated micropores of a columnar supramolecular solid. Angew. Chem. Int. Ed. 2006, 45, 7030–7033. [Google Scholar] [CrossRef]
- Taylor, T.J.; Gabbaï, F.P. Supramolecular stabilization of α,ω-diphenylpolyynes by complexation to the tridentate lewis acid [o-C6F4Hg]3. Organometallics 2006, 25, 2143–2147. [Google Scholar] [CrossRef]
- Gunawardana, C.A.; Aakeröy, C.B. Co-crystal synthesis: Fact, fancy, and great expectations. Chem Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef]
- Maiti, B.; Bhattacharjee, S.; Bhattacharya, S. Perfluoroarene induces a pentapeptidic hydrotrope into a pH-tolerant hydrogel allowing naked eye sensing of Ca2+ ions. Nanoscale 2019, 11, 2223–2230. [Google Scholar] [CrossRef]
- Thalladi, V.R.; Weiss, H.-C.; Blaser, D.; Boese, R.; Nangia, A.; Desiraju, G.R. C-H···F interactions in the crystal structures of some fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar] [CrossRef]
- Ferreira da Silva, J.L.; Shimizu, K.; Duarte, M.T. The role of halogen interactions in the crystal structure of biscyclopentadiethnyl dihalides. CrystEngComm 2017, 19, 2802–2812. [Google Scholar] [CrossRef]
- Marushima, Y.; Uchiumi, Y.; Ogua, K.; Hori, A. Intermolecular π-stacking and F···F interactions of fluorine-substituted meso-alkynylporphyrin. Acta Cryst. 2010, C66, o406–o409. [Google Scholar]
- Hori, A.; Shinohe, A.; Yamasaki, M.; Nishibori, E.; Aoyagi, S.; Sakata, M. 1:1 Cross-assembly of two β-diketonate complexes through arene-perfluoroarene interactions. Angew. Chem. Int. Ed. 2007, 46, 7617–7620. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Arii, T. Cation-π and arene-perfluoroarene interactions between Cu(II) fluorine-substituted β-diketonate complex and benzenes. CrystEngComm 2007, 9, 215–217. [Google Scholar] [CrossRef]
- Hori, A.; Takatani, S.; Miyamoto, T.K.; Hasegawa, M. Luminescence from π–π stacked bipyridines through arene–perfluoroarene interactions. CrystEngComm 2009, 11, 567–569. [Google Scholar] [CrossRef]
- Hori, A.; Nakajima, K.; Akimoto, Y.; Naganuma, K.; Yuge, H. Guest-adjusted encapsulation and thermal studies of non-porous mononuclear Cu(II) coordination complexes through electrostatic interactions induced by fluorine substitution. CrystEngComm 2014, 16, 8805–8817. [Google Scholar] [CrossRef]
- Hori, A.; Gonda, R.; Rzeznicka, I.I. Enhanced adsorption of small gas molecules in metal (Cu2+, Pd2+, Pt2+) complexes induced by ligand fluorination. CrystEngComm 2017, 19, 6263–6266. [Google Scholar] [CrossRef]
- Hori, A.; Shinohe, A.; Takatani, S.; Miyamoto, T.K. Synthesis and crystal structures of fluorinated β-diketonate metal (Al3+, Co2+, Ni2+, and Cu2+) complexes. Bull. Chem. Soc. Jpn. 2009, 82, 96–98. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Gao, S.; Wang, Z.-M.; Liao, C.-S.; Yan, C.-H.; Xu, G.-X. Synthesis and structure of bis(dibenzoylmethanato)copper(II). J. Chem. Cryst. 1999, 29, 793–796. [Google Scholar] [CrossRef]
- Morita, H.; Nakanishi, H. Electronic structure and spectra of the enol form of some β-diketones. Bull. Chem. Soc. Jpn. 1981, 54, 378–386. [Google Scholar] [CrossRef]
- Moriyasu, M.; Kato, A.; Hashimoto, Y. Kinetic studies of fast equilibrium by means of high-performance liquid chromatography. Part 11. Keto-enol tautomerism of some β-dicarbonyl compounds. J. Chem. Soc. Perkin Trans. 1986, 2, 515–520. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Evidence for resonance-assisted hydrogen bonding. 2. Intercorrelation between crystal structure and spectroscopic parameters in eight intramolecularly hydrogen bonded 1,3-diaryl-1,3-propanedione enols. J. Am. Chem. Soc. 1991, 113, 4917–4925. [Google Scholar] [CrossRef]
- Gilli, P.; Bertolasi, V.; Pretto, L.; Ferretti, V.; Gilli, G. Covalent versus electrostatic nature of the strong hydrogen bond: discrimination among single, double, and asymmetric single-well hydrogen bonds by variable-temperature X-ray crystallographic methods in β-diketone enol RAHB systems. J. Am. Chem. Soc. 2004, 126, 3845–3855. [Google Scholar] [CrossRef]
- Tobita, S.; Ohba, J.; Nakagawa, K.; Shizuka, H. Recovery mechanism of the reaction intermediate produced by photoinduced cleavage of the intramolecular hydrogen bond of dibenzoylmethane. J. Photochem. Photobiol. A Chem. 1995, 92, 61–67. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J. Photochemical studies of 4-tert-butyl-4′-methoxydibenzoylmethane (BM-DBM). J. Photochem. Photobiol. B Biol. 2001, 64, 117–122. [Google Scholar] [CrossRef]
- Nagashima, N.; Kudoh, S.; Takayanagi, M.; Nakata, M. UV-induced photoisomerization of acetylacetone and identification of less-stable isomers by low-temperature matrix-isolation infrared spectroscopy and density functional theory calculation. J. Phys. Chem. A 2001, 105, 10832–10838. [Google Scholar] [CrossRef]
- Wiechmann, M.; Port, H.; Frey, W.; Laermer, F.; Elsaesser, T. Time-resolved spectroscopy on ultrafast proton transfer in 2-(2′-hydroxy-5′-methylphenyl)benzotriazole in liquid and polymer environments. J. Phys. Chem. 1991, 95, 1918–1923. [Google Scholar] [CrossRef]
- Ünver, H.; Kabak, M.; Zengin, D.M.; Durlu, T.N. Keto-enal tautomerism, conformations, and structure of 1-[N-(4-chlorophenyl)]aminomethhylidene-2(1H)naphthalenone. J. Chem. Crystallogr. 2001, 31, 203–209. [Google Scholar] [CrossRef]
- Claramunt, R.M.; López, C.; María, M.D.S.; Sanz, D.; Elguero, J. The use of NMR spectroscopy to study tautomerism. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 169–206. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Filler, R.; Rao, Y.S.; Biezais, A.; Miller, F.N.; Beaucaire, V.D. Polyfluoroaryl β-dicarbonyl compounds. J. Org. Chem. 1970, 35, 930–935. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Thomas, L.H.; Florence, A.J.; Wilson, C.C. Hydrogen atom behavior imaged in a short intramolecular hydrogen bond using the combined approach of X-ray and neutron diffraction. New. J. Chem. 2009, 33, 2486–2490. [Google Scholar] [CrossRef]
- Cea-Olivares, R.; Rodriguez, I.; Rosales, M.J.; Toscano, R.A. The structure of triketones in the solid sate. The crystal structure of 1,5-diphenylpentane-1,3,5-trione. Aust. J. Chem. 1987, 40, 1127–1130. [Google Scholar] [CrossRef]
- Wheeler, S.E. Local nature of Substituent effects in stacking interactions. J. Am. Chem. Soc. 2011, 133, 3687–3689. [Google Scholar] [CrossRef] [PubMed]
- Ringer, A.L.; Sherrill, C.D. Substituent Effects in Sandwich Configurations of Multiply Substituted Benzene Dimers Are Not Solely Governed By Electrostatic Control. J. Am. Chem. Soc. 2009, 131, 4574–4575. [Google Scholar] [CrossRef] [PubMed]







| H1 | H22 | H1•H3 | H22•H24 | |
| Chemical formula | C15H2F10O2 | C17H4F10O3 | C30H14F10O4 | C34H18F10O6 |
| Formula weight | 404.17 | 446.20 | 628.41 | 712.48 |
| Crystal system | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | P21/n | P21/c | P21/c | P21/c |
| a [Å] | 10.3800(11) | 17.4336(12) | 13.4003(10) | 15.7397(14) |
| b [Å] | 5.6285(6) | 4.9910(3) | 7.0784(5) | 7.0887(6) |
| c [Å] | 23.196(3) | 18.3069(12) | 26.1345(18) | 25.835(2) |
| β [°] | 94.2233(12) | 106.929(1) | 95.2152(8) | 98.842(1) |
| V [Å3] | 1351.5(2) | 1523.88(17) | 2468.7(3) | 2848.3(4) |
| Z | 4 | 4 | 4 | 4 |
| Dc [Mg m−3] | 1.986 | 1.945 | 1.691 | 1.662 |
| μ [mm−1] | 0.223 | 0.213 | 0.162 | 0.156 |
| F(000) | 792 | 880 | 1264 | 1440 |
| Rint | 0.0240 | 0.0194 | 0.0238 | 0.0265 |
| GOF | 1.061 | 1.033 | 1.047 | 1.035 |
| R [(I) > 2σ (I)] | 0.0303 | 0.0301 | 0.0337 | 0.0334 |
| wR (Fo2) | 0.0845 | 0.0849 | 0.0930 | 0.0952 |
| CCDC No. | 1827049 | 1827050 | 1827051 | 1827052 |
| H1•H3 | H22•H24 | |
| Intramolecular hydrogen bonds (O-H···O) | O1···O2, 2.5313(15) Å | O1···O2, 2.5495(14) Å |
| O2···O3, 2.5811(14) Å | ||
| O3···O4, 2.4882(15) Å | O4···O5, 2.5584(14) Å | |
| O5···O6, 2.5632(14) Å | ||
| O1···H2-O2, 147° | O1-H1···O2, 147° | |
| O2···H3-O3, 146° | ||
| O3···H4-O4, 148° | O4-H4···O5, 146° | |
| O5···H6-O6, 147° | ||
| Intermolecular hydrogen bonds (O-H···O) | not found | not found |
| Arene-perfluoroarene [Cg(C6F5)···Cg(C6H5)] | π(C1-6)···π(C29-34), 3.6671(8) Å | π(C1-6)···π(C29-34), 3.6949(8) Å |
| π(C10-15)···π(C16-21), 4.1008(9) Å | π(C12-17)···π(C18-23), 4.0439(8) Å | |
| C-F···π [C-F···Cg(C6F5)] | C4-F4···π(C29-34), 3.4806(11) Å | C4-F4···π(C29-34), 3.4959(11) Å |
| C14-F9···π(C16-21), 3.2877(11) Å | C16-F9···π(C18-23), 3.2621(11) Å | |
| C15-F10···π(C16-21), 3.2364(10) Å | C17-F10···π(C18-23), 3.3073(11) Å |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusakawa, T.; Sakai, S.; Nakajima, K.; Yuge, H.; Rzeznicka, I.I.; Hori, A. Synthesis, Structures and Co-Crystallizations of Perfluorophenyl Substituted β-Diketone and Triketone Compounds. Crystals 2019, 9, 175. https://doi.org/10.3390/cryst9030175
Kusakawa T, Sakai S, Nakajima K, Yuge H, Rzeznicka II, Hori A. Synthesis, Structures and Co-Crystallizations of Perfluorophenyl Substituted β-Diketone and Triketone Compounds. Crystals. 2019; 9(3):175. https://doi.org/10.3390/cryst9030175
Chicago/Turabian StyleKusakawa, Takumi, Shunichiro Sakai, Kyosuke Nakajima, Hidetaka Yuge, Izabela I. Rzeznicka, and Akiko Hori. 2019. "Synthesis, Structures and Co-Crystallizations of Perfluorophenyl Substituted β-Diketone and Triketone Compounds" Crystals 9, no. 3: 175. https://doi.org/10.3390/cryst9030175
APA StyleKusakawa, T., Sakai, S., Nakajima, K., Yuge, H., Rzeznicka, I. I., & Hori, A. (2019). Synthesis, Structures and Co-Crystallizations of Perfluorophenyl Substituted β-Diketone and Triketone Compounds. Crystals, 9(3), 175. https://doi.org/10.3390/cryst9030175