A 1,6-Diphenylpyrene-Based, Photoluminescent Cyclophane Showing a Nematic Liquid-Crystalline Phase at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Method
2.2. Synthesis
3. Results and Discussion
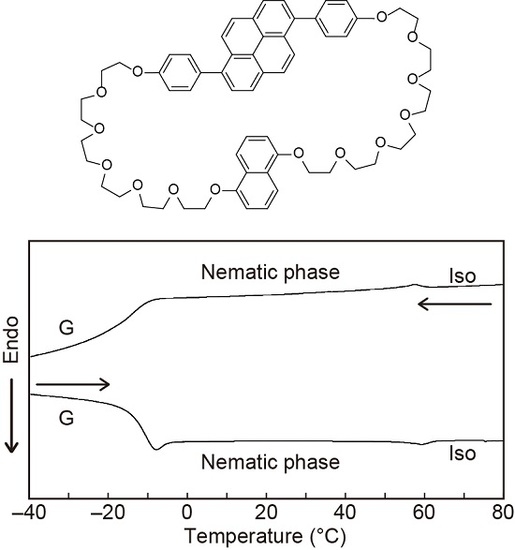
3.1. Molecular Designs
3.2. LC Properties
3.3. Absorption and Photoluminescence Properties
3.4. Photoluminescence Properties of 1 in the Nematic Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chen, J.; Zhu, W.; Baranoff, E. Recent progress in luminescent liquid crystal materials: Design, properties and application for linearly polarised emission. J. Mater. Chem. C 2015, 3, 7993–8005. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073–1095. [Google Scholar] [CrossRef]
- Yamane, S.; Tanabe, K.; Sagara, Y.; Kato, T. Stimuli-responsive photoluminescent liquid crystals. Top. Curr. Chem. 2012, 318, 395–405. [Google Scholar]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P.; Vill, V. Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Vill, V., Eds.; WileyVCH: Weinheim, Germany, 2014. [Google Scholar]
- Singh, G.; Vijaya Prakash, G.; Choudhary, A.; Biradar, A.M. Homeotropic alignment of nematic liquid crystals with negative dielectric anisotropy. Phys. B Condens. Matter 2010, 405, 2118–2121. [Google Scholar] [CrossRef]
- Kato, T.; Yasuda, T.; Kamikawa, Y.; Yoshio, M. Self-assembly of functional columnar liquid crystals. Chem. Commun. 2009, 0, 729–739. [Google Scholar] [CrossRef]
- Funahashi, M. Nanostructured liquid-crystalline semiconductors—A new approach to soft matter electronics. J. Mater. Chem. C 2014, 2, 7451–7459. [Google Scholar] [CrossRef]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Ordered materials for organic electronics and photonics. Adv. Mater. 2011, 23, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Kawasumi, M.; Rinaldi, P.L.; Litman, V.E. Synthesis and characterization of cyclic liquid crystalline oligomers based on 1-(4-hydroxy-4’-biphenylyl)-2-(4-hydroxyphenyl)butane and 1,10-dibromodecane. Macromolecules 1992, 25, 3851–3861. [Google Scholar] [CrossRef]
- Percec, V.; Turkaly, P.J.; Asandei, A.D. Macrocyclization Overrides the Polymer Effect in the Stabilization of Liquid Crystalline (LC) Phases with a Novel Odd−Even Alternation. A Demonstration with LC Crown Ethers. Macromolecules 1997, 30, 943–952. [Google Scholar] [CrossRef]
- Percec, V.; Asandei, A.D.; Ungar, G. From Regioirregular Linear Main-Chain Liquid-Crystal Polyethers Exhibiting Two Uniaxial Nematic Phases to Macrocyclic Main-Chain Oligoethers Displaying Nematic and Smectic Phases. Chem. Mater. 1996, 8, 1550–1557. [Google Scholar] [CrossRef]
- Percec, V.; Asandei, A.D.; Chu, P. Design of Side Chain and Main Chain Liquid Crystalline Polymers Containing Supramolecular Quasi-Rigid-Rodlike Mesogens Obtained from Collapsed Main Chain Macrocyclics. Macromolecules 1996, 29, 3736–3750. [Google Scholar] [CrossRef]
- Percec, V.; Kawasumi, M. Liquid-crystalline polyethers based on conformational isomerism. 32. Effect of molecular weight on the phase behavior of linear and macrocyclic oligoethers and of linear polyethers based on 1-(4-hydroxy-4’-biphenylyl)-2-(4-hydroxyphenyl)butane and 1,10-dibromodecane. Macromolecules 1993, 26, 3663–3675. [Google Scholar]
- Hegmann, T.; Neumann, B.; Wolf, R.; Tschierske, C. Liquid crystalline paracyclophanes and ansa compounds—series of polyether macrocycles incorporating diacetylene, phenyl, biphenyl, p-terphenyl and 2,5-diphenyl-1,3,4-thiadiazole rigid cores. J. Mater. Chem. 2005, 15, 1025–1034. [Google Scholar] [CrossRef]
- Neumann, B.; Hegmann, T.; Wagner, C.; Ashton, P.R.; Wolf, R.; Tschierske, C. Liquid crystalline macrocycles containing phenylpyrimidine units. J. Mater. Chem. 2003, 13, 778–784. [Google Scholar] [CrossRef]
- Neumann, B.; Hegmann, T.; Wolf, R.; Tschierske, C. Binuclear cyclopalladated cyclophanes: Towards a new family of metallomesogens. Chem. Commun. 1998, 105–106. [Google Scholar] [CrossRef]
- Neumann, B.; Joachimi, D.; Tschierske, C. Liquid crystalline macrocycles: Novel glass-forming nematic materials that can undergo charge transfer induced phase transitions. Adv. Mater. 1997, 9, 241–244. [Google Scholar] [CrossRef]
- Sagara, Y.; Tamaoki, N.; Fukuhara, G. Cyclophane-Based Fluorescence Tuning Induced by Hydrostatic Pressure Changes. ChemPhotoChem 2018, 2, 959–963. [Google Scholar] [CrossRef]
- Sagara, Y.; Seki, A.; Kim, Y.; Tamaoki, N. Linearly polarized photoluminescence from an asymmetric cyclophane showing thermo- and mechanoresponsive luminescence. J. Mater. Chem. C 2018, 6, 8453–8459. [Google Scholar] [CrossRef]
- Mase, K.; Sasaki, Y.; Sagara, Y.; Tamaoki, N.; Weder, C.; Yanai, N.; Kimizuka, N. Stimuli-Responsive Dual-Color Photon Upconversion: A Singlet-to-Triplet Absorption Sensitizer in a Soft Luminescent Cyclophane. Angew. Chem. Int. Ed. 2018, 57, 2806–2810. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Weder, C.; Tamaoki, N. Asymmetric Cyclophanes Permit Access to Supercooled Nematic Liquid Crystals with Stimulus-Responsive Luminescence. Chem. Mater. 2017, 29, 6145–6152. [Google Scholar] [CrossRef]
- Sagara, Y.; Tamaoki, N. Mechanoresponsive luminescence and liquid-crystalline behaviour of a cyclophane featuring two 1,6-bis(phenylethynyl)pyrene groups. RSC Adv. 2017, 7, 47056–47062. [Google Scholar] [CrossRef]
- Sagara, Y.; Weder, C.; Tamaoki, N. Tuning the thermo- and mechanoresponsive behavior of luminescent cyclophanes. RSC Adv. 2016, 6, 80408–80414. [Google Scholar] [CrossRef]
- Sagara, Y.; Simon, Y.C.; Tamaoki, N.; Weder, C. A mechano- and thermoresponsive luminescent cyclophane. Chem. Commun. 2016, 52, 5694–5697. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Karman, M.; Verde-Sesto, E.; Matsuo, K.; Kim, Y.; Tamaoki, N.; Weder, C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Calvino, C.; Sagara, Y.; Buclin, V.; Haehnel, A.P.; del Prado, A.; Aeby, C.; Simon, Y.C.; Schrettl, S.; Weder, C. Mechanoresponsive, Luminescent Polymer Blends Based on an Excimer-Forming Telechelic Macromolecule. Macromol. Rapid Commun. 2018, 40, 1800705. [Google Scholar] [CrossRef] [PubMed]
- Haehnel, A.P.; Sagara, Y.; Simon, Y.C.; Weder, C. Mechanochemistry in Polymers with Supramolecular Mechanophores. Top. Curr. Chem. 2015, 369, 345–375. [Google Scholar]
- Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. Material Design for Piezochromic Luminescence: Hydrogen-Bond-Directed Assemblies of a Pyrene Derivative. J. Am. Chem. Soc. 2007, 129, 1520–1521. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Z.; Teng, M.; Xu, Z.; Jia, X. Mechanically Induced Multicolor Change of Luminescent Materials. Chemphyschem 2015, 16, 1811–1828. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, Z.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in mechanochromic luminescent metal complexes. J. Mater. Chem. C 2013, 1, 3376–3390. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L. Dynamic crystals: Visually detected mechanochemical changes in the luminescence of gold and other transition-metal complexes. Angew. Chem. Int. Ed. 2009, 48, 2641–2644. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Honda, K.; Kurosawa, S.; Tanaka, M. Dynamic Properties of Self-Assembled Monolayers of Mercapto Oligo(ethylene oxide) Methyl Ether on an Oscillating Solid–Liquid Interface. J. Phys. Chem. C 2014, 118, 16067–16073. [Google Scholar] [CrossRef]
- Suneesh, C.V.; Gopidas, K.R. Long-Lived Photoinduced Charge Separation Due to the Inverted Region Effect in 1,6-Bis(phenylethynyl)pyrene−Phenothiazine Dyad. J. Phys. Chem. C 2010, 114, 18725–18734. [Google Scholar] [CrossRef]
- Sagara, Y.; Komatsu, T.; Ueno, T.; Hanaoka, K.; Kato, T.; Nagano, T. Covalent attachment of mechanoresponsive luminescent micelles to glasses and polymers in aqueous conditions. J. Am. Chem. Soc. 2014, 136, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Maeda, T.; Mizuno, K.; Fujimoto, K.; Shimizu, H.; Inouye, M. Alkynylpyrenes as Improved Pyrene-Based Biomolecular Probes with the Advantages of High Fluorescence Quantum Yields and Long Absorption/Emission Wavelengths. Chem. Eur. J. 2006, 12, 824–831. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagara, Y.; Muramatsu, T.; Tamaoki, N. A 1,6-Diphenylpyrene-Based, Photoluminescent Cyclophane Showing a Nematic Liquid-Crystalline Phase at Room Temperature. Crystals 2019, 9, 92. https://doi.org/10.3390/cryst9020092
Sagara Y, Muramatsu T, Tamaoki N. A 1,6-Diphenylpyrene-Based, Photoluminescent Cyclophane Showing a Nematic Liquid-Crystalline Phase at Room Temperature. Crystals. 2019; 9(2):92. https://doi.org/10.3390/cryst9020092
Chicago/Turabian StyleSagara, Yoshimitsu, Tatsuya Muramatsu, and Nobuyuki Tamaoki. 2019. "A 1,6-Diphenylpyrene-Based, Photoluminescent Cyclophane Showing a Nematic Liquid-Crystalline Phase at Room Temperature" Crystals 9, no. 2: 92. https://doi.org/10.3390/cryst9020092
APA StyleSagara, Y., Muramatsu, T., & Tamaoki, N. (2019). A 1,6-Diphenylpyrene-Based, Photoluminescent Cyclophane Showing a Nematic Liquid-Crystalline Phase at Room Temperature. Crystals, 9(2), 92. https://doi.org/10.3390/cryst9020092