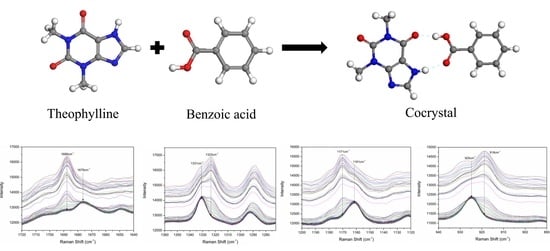
Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Theophylline-Benzoic Acid Cocrystal
2.3. Monitoring Cocrystallization Process of Theophylline and Benzoic Acid in Slurry Crystallization
2.4. Analytical Methods
3. Results and Discussion
3.1. Solid Phases Characterization of Theophylline-benzoic Acid Cocrystal
3.2. Crystal Structure Analysis of TP-BA Cocrystal
3.3. On-Line Monitoring of TP-BA Cocrystal Formation Process in Slurry Crystallization
3.4. Influence of Suspension Density of Raw Materials and Temperature on Theophylline-benzoic Acid Cocrystal Formation in Slurry Crystallization
3.5. On-Line Monitoring of TP-BA Cocrystal Formation Process in Cooling Crystallization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Byrn, S.R.; Zografi, G.; Chen, X. Accelerating proof of concept for small molecule drugs using solid-state chemistry. J. Pharm. Sci. 2010, 99, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Rodríguez-Hornedo, N. Cocrystals: Molecular design of pharmaceutical materials. Mol. Pharm. 2007, 4, 299–300. [Google Scholar] [CrossRef]
- Liao, X.M.; Gautam, M.; Grill, A.; Zhu, H.J.J. Effect of position isomerism on the formation and physicochemical properties of pharmaceutical co-crystals. J. Pharm. Sci. 2010, 99, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Thanigaiman, K.; Khalib, N.C.; Temel, E.; Arshad, S.; Razak, I.A. New supramolecular cocrystal of 2-amino-5-chloropyridine with 3-methylbenzoic acids: Syntheses, structural characterization, hirshfeld surfaces and quantum chemical investigations. J. Mol. Struct. 2015, 1099, 246–256. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S.; et al. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef]
- Remenar, J.F.; Perterson, M.L.; Stephens, P.W.; Zhang, Z.; Zimenkov, Y.; Hickey, M.B. Celecoxib: Nicotinamide dissociation: Using excipients to capture the cocrystal’s potential. Mol. Pharm. 2007, 4, 386–400. [Google Scholar] [CrossRef]
- Zhang, S.; Rasmuson, Å.C. The theophylline–oxalic acid co-crystal system: Solid phases, thermodynamics and crystallization. CrystEngComm 2012, 14, 4644–4655. [Google Scholar] [CrossRef]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. In situ investigation of a self-accelerated cocrystal formation by grinding pyrazinamide with oxalic acid. Molecules 2016, 21, 917. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Indomethacin-saccharin cocrystal: Design, synthesis and preliminary pharmaceutical characterization. Pharm. Res. 2008, 25, 530–541. [Google Scholar] [CrossRef]
- Chieng, N.; Rades, T.; Aaltonen, J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2011, 55, 618–644. [Google Scholar] [CrossRef]
- Holaň, J.; Štěpánek, F.; Billot, P.; Ridvan, L. The construction, prediction and measurement of co-crystal ternary phase diagrams as a tool for solvent selection. Eur. J. Pharm. Sci. 2014, 63, 124–131. [Google Scholar] [CrossRef]
- Ueto, T.; Takata, N.; Muroyama, N.; Nedu, A.; Sasaki, A.; Tanida, S.; Terada, K. Polymorphs and a hydrate of furosemide-nicotinamide 1:1 cocrystal. Cryst. Growth Des. 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Soares, F.L.F.; Carneiro, R.L. Green Synthesis of Ibuprofen−Nicotinamide Cocrystals and In-Line Evaluation by Raman Spectroscopy. Cryst. Growth Des. 2013, 13, 1510–1517. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, K.J.; Ulrich, J. In Situ Monitoring of Cocrystallization of Salicylic Acid−4,4′-Dipyridyl in Solution Using Raman Spectroscopy. Cryst. Growth Des. 2014, 14, 2893–2899. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, P.; Dang, L.; Wei, H. Monitoring of cocrystallization of ethenzamide saccharin: Insight into kineticprocess by in situ Raman spectroscopy. Chem. Eng. Res. Des. 2016, 109, 249–257. [Google Scholar] [CrossRef]
- Kojima, T.; Tsutsumi, S.; Yamamoto, K.; Ikeda, Y.; Moriwaki, T. High-throughput cocrystal slurry screening by use of in situ Raman microscopy and multi-well plate. Int. J. Pharm. 2010, 399, 52–59. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef]
- Abourahma, H.; Urban, J.M.; Morozowich, N.; Chan, B. Examining the robustness of a theophylline cocrystal during grinding with additives. CrystEngComm 2014, 14, 6163–6169. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline Cocrystals Prepared by Spray Drying: Physicochemical Properties and Aerosolization Performance. AAPS PharmSciTech. 2013, 14, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Fulias, A.; Soica, C.; Ledeti, I.; Vlase, T.; Vlase, G.; Suta, L.M.; Belu, I. Characterization of Pharmaceutical Acetylsalicylic Acid-theophylline Cocrystal Obtained by Slurry Method Under Microwave Irradiation. Rev. Chim. 2014, 65, 1281–1284. [Google Scholar]
- Lin, H.L.; Hsu, P.C.; Lin, S.Y. Theophylline-citric acid co-crystals easily induced by DSC-FTIR microspectroscopy or different storage conditions. Asian J. Pharm. Sci. 2013, 8, 18–26. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and Characterization of Theophylline-Nicotinamide Cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Rasmuson, Å.C. Thermodynamics and crystallization of a theophylline-salicylic acid cocrystal. CrystEngComm 2015, 17, 4125–4135. [Google Scholar] [CrossRef]
- Heiden, S.; Tröbs, L.; Wenzel, K.J.; Emmerling, F. Mechanochemical synthesis and structural characterisation of a theophylline-benzoic acid cocrystal (1:1). CrystEngComm 2012, 14, 5128–5129. [Google Scholar] [CrossRef]
- Zhang, S.; Rasmuson, Å.C. Thermodynamics and Crystallization of the Theophylline-Glutaric Acid Cocrystal. Cryst. Growth Des. 2013, 13, 1153–1161. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant. 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Rahim, S.A.; Hammond, R.B.; Roberts, K.J. Scalable solution cocrystallization: Case of carbamazepine-nicotinamide I. CrystEngComm 2009, 11, 501–509. [Google Scholar] [CrossRef]







| Chemical | Source | Mass Fraction Purity | Purification Method |
|---|---|---|---|
| Theophylline | Aladdin-Reagent Technology Co. Ltd. (Shanghai, China) | >0.990 | GCa |
| Benzoic acid | Tianjin Guangfu Chemical Reagent Co. (Tianjin, China) | >0.990 | GCa |
| Deionized water | Tianjin Kewei Chemical Co. Ltd. (Tianjin, China) | >0.995 | GCa |
| Methanol | Tianjin Kewei Chemical Co. Ltd. (Tianjin, China) | >0.995 | GCa |
| Total Spectra Number | Total Recording Time/min | Time Point of First Spectra/min | Time Interval/min |
|---|---|---|---|
| 20 | 100 | 5 | 5 |
| Temperature/K | Initial Concentration of TP/M | Initial Concentration of BA/M | Suspension Density of TP/M | Suspension Density of BA/M | Ratio of TP and BA in Cocrystal a | Formation Time/min | |
|---|---|---|---|---|---|---|---|
| exp 1 | 298.15 | 0.165 | 0.165 | 0.078 | 0.078 | 1:1 | 44 |
| exp 2 | 298.15 | 0.130 | 0.130 | 0.042 | 0.042 | 1:1 | 57 |
| exp 3 | 313.15 | 0.292 | 0.292 | 0.078 | 0.078 | 1:1 | 22 |
| exp 4 | 313.15 | 0.256 | 0.256 | 0.042 | 0.042 | 1:1 | 43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhou, L.; Yang, W.; Li, Y.; Yang, Y.; Zhang, Z.; Wang, C.; Zhang, X.; Yin, Q. Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy. Crystals 2019, 9, 329. https://doi.org/10.3390/cryst9070329
Huang Y, Zhou L, Yang W, Li Y, Yang Y, Zhang Z, Wang C, Zhang X, Yin Q. Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy. Crystals. 2019; 9(7):329. https://doi.org/10.3390/cryst9070329
Chicago/Turabian StyleHuang, Yaohui, Ling Zhou, Wenchao Yang, Yang Li, Yongfan Yang, Zaixiang Zhang, Chang Wang, Xia Zhang, and Qiuxiang Yin. 2019. "Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy" Crystals 9, no. 7: 329. https://doi.org/10.3390/cryst9070329
APA StyleHuang, Y., Zhou, L., Yang, W., Li, Y., Yang, Y., Zhang, Z., Wang, C., Zhang, X., & Yin, Q. (2019). Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy. Crystals, 9(7), 329. https://doi.org/10.3390/cryst9070329