Induced Nucleation of Biomimetic Nanoapatites on Exfoliated Graphene Biomolecule Flakes by Vapor Diffusion in Microdroplets
Abstract
:1. Introduction
2. Materials and Methods
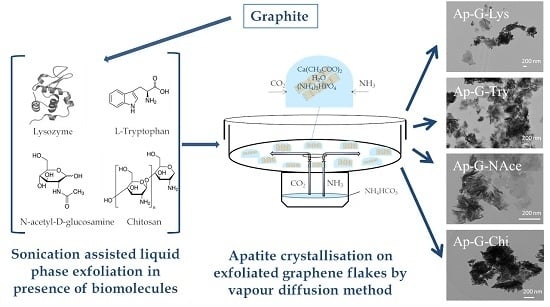
2.1. Exfoliated Graphene Flakes
2.2. Precipitation Method
2.3. Characterization
2.4. The Cytocompatibility of Graphene–Apatite Nanocomposites
3. Results and Discussion
3.1. Crystallographic and Spectroscopic Features of the Nanocomposites
3.2. Morphological Characteristics of Hybrid Apatite–Graphene Nanocomposites
3.3. Cytocompatibility of Hybrid Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gómez–Morales, J.; Iafisco, M.; Delgado–López, J.M.; Sarda, S.; Druet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- De Groot, K.; Geesink, R.; Klein, C.P.A.T.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Pichugin, V.F.; Eshenko, E.V.; Surmenev, R.A.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Ryabtseva, M.A.; Sokhoreva, V.V.; Khlusov, I.A. Application of high-frequency magnetron sputtering to deposit thin calcium-phosphate biocompatible coatings on a Titanium surface. J. Surf. Invest. X.-ray, Synchr. Neutr. Tech. 2007, 1, 679–682. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghan, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mechan. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Bosco, R.; Leeuwenburgh, R.S.C.G.; van den Beucken, J.J.J.P.; Jansen, J.A.; Prat, M.; Roveri, N. Electrostatic spray deposition of biomimetic nanocrystalline apatite coatings onto titanium. Adv. Eng. Mater. 2012, 14, B13–B20. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Rodríguez-Clemente, R.; Armas, B.; Combescure, C.; Berjoan, R.; Cubo, J.; Martínez, E.; García-Carmona, J.; Garelik, S.; Murtra, J.; et al. Controlled nucleation and growth of thin hydroxyapatite layers on titanium implants by using induction heating technique. Langmuir 2004, 20, 5174–5178. [Google Scholar] [CrossRef]
- Zhou, S.; Zheng, X.; Yu, X.; Wang, J.; Weng, J.; Li, X.; Feng, B.; Yin, M. Hydrogen Bonding Interaction of Poly(d,l-Lactide)/hydroxyapatite nanocomposites. Chem. Mater. 2007, 19, 247–253. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shin, K.-H.; Koh, Y.-H.; Hah, M.J.; Moon, J.; Kim, H.-E. Production of Poly(ε--Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity. Materials 2017, 10, 1123. [Google Scholar] [CrossRef]
- Li, J.; Fartash, B.; Hermansson, L. Hydroxyapatite-alumina composites and bone-bonding. Biomaterials. 1995, 16, 417–422. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M. Hydroxyapatite–carbon nanotube composites for biomedical applications: A review. Int. J. Appl. Ceram. Technol. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.; Hone, V. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Westervelt, R.M. Graphene nanoelectronics. Science 2008, 320, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Iafisco, M.; Gomez-Morales, J.; Hernandez-Hernandez, M.A.; García-Ruiz, J.M.; Roveri, N. Biomimetic Carbonate–Hydroxyapatite Nanocrystals Prepared by Vapor Diffusion. Adv. Eng. Mater. 2010, 12, 218–223. [Google Scholar]
- Gomez-Morales, J.; Delgado-Lopez, J.M.; Iafisco, M.; Hernandez-Hernandez, M.A.; Prat, M. Amino acidic control of calcium phosphate precipitation by using the vapor diffusion method in microdroplets. Cryst. Growth Des. 2011, 11, 4802–4809. [Google Scholar] [CrossRef]
- Nassif, N.; Martineau, F.; Syzgantseva, O.; Gobeaux, F.; Willinger, M.; Coradin, T.; Cassaignon, S.; Azais, T.; Giraud-Guille, M.M. In Vivo Inspired Conditions to Synthesize Biomimetic Hydroxyapatite. Chem. Mater. 2010, 22, 3653–3663. [Google Scholar] [CrossRef]
- Gomez-Morales, J.; Hernandez-Hernandez, A.; Sazaki, G.; Garcia-Ruiz, J.M. Nucleation and Polymorphism of Calcium Carbonate by a Vapor Diffusion Sitting Drop Crystallization Technique. Cryst. Growth Des. 2010, 10, 963–969. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.; Rodriguez-Navarro, A.B.; Gomez-Morales, J.; Jimenez-Lopez, C.; Nys, Y.; Garcia-Ruiz, J.M. Influence of model globular proteins with different isoelectric points on the precipitation of calcium carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.; Gómez-Morales, J.; Rodriguez-Navarro, A.B.; Gautron, J.; Nys, Y.; Garcia-Ruiz, J.M. Identification of some active proteins on the process of hen eggshell formation. Cryst. Growth Des. 2008, 8, 4330–4339. [Google Scholar] [CrossRef]
- Calvaresi, M.; DiGiosia, M.; Ianiro, A.; Valle, F.; Fermani, S.; Polishchuk, I.; Pokroy, B.; Falini, G. Morphological changes of calcite single crystals induced by graphene–biomolecule adducts. J. Cryst. Growth 2017, 457, 356–361. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Gavira-Gallardo, J.A. Bioinspired calcium phosphate coated mica sheets by vapor diffusion and its effects on lysozyme assembly and crystallization. Cryst. Growth Des. 2016, 16, 5150–5158. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Giordano, S.; Ponzetto, C.; Di Renzo, M.F.; Cooper, C.S.; Comoglio, P.M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 1989, 339, 155–156. [Google Scholar] [CrossRef]
- Zamperone, A.; Pietronave, S.; Merlin, S.; Colangelo, D.; Ranaldo, G.; Medico, E.; di Scipio, F.; Berta, G.N.; Follenzi, A.; Prat, M. Isolation and characterization of a spontaneously immortalized multipotent mesenchymal cell line derived from mouse subcutaneous adipose tissue. Stem Cells Dev. 2013, 22, 2873–2884. [Google Scholar] [CrossRef]
- Delgado-Lopez, J.M.; Iafisco, M.; Rodriguez-Ruiz, I.; Tampieri, A.; Prat, M.; Gómez–Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef]
- Iafisco, M.; Marchetti, M.; Gómez-Morales, J.; Hernández-Hernández, M.A.; García-Ruiz, J.M.; Roveri, N. Silica gel template for calcium phosphates crystallization. Cryst. Growth Des. 2009, 9, 4912–4921. [Google Scholar] [CrossRef]
- Blanton, T.N.; Majumdar, D. X-ray diffraction characterization of polymer intercalated graphite oxide. Powder Diffr. 2012, 27, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Kadir, M.F.Z.; Aspanut, Z.; Majid, S.R.; Arof, A.K. FTIR studies of plasticized poly(vinylalcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim. Acta Mol. Biomol. Spectros. 2011, 78, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and postsynthetic parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Comprehensive Biomaterials 2014, 187–221.
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies ofsynthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T.L. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Guardia, L.; Fernandez-Merino, M.J.; Paredes, J.I.; Solis-Fernandez, P.; Villar Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef] [Green Version]
- Iafisco, M.; Ramírez-Rodríguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gómez-Morales, J.; Delgado-López, J.M. The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 2015, 17, 507–515. [Google Scholar] [CrossRef]
- ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 22 December 2018).





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Morales, J.; González-Ramírez, L.A.; Verdugo-Escamilla, C.; Fernández Penas, R.; Oltolina, F.; Prat, M.; Falini, G. Induced Nucleation of Biomimetic Nanoapatites on Exfoliated Graphene Biomolecule Flakes by Vapor Diffusion in Microdroplets. Crystals 2019, 9, 341. https://doi.org/10.3390/cryst9070341
Gómez-Morales J, González-Ramírez LA, Verdugo-Escamilla C, Fernández Penas R, Oltolina F, Prat M, Falini G. Induced Nucleation of Biomimetic Nanoapatites on Exfoliated Graphene Biomolecule Flakes by Vapor Diffusion in Microdroplets. Crystals. 2019; 9(7):341. https://doi.org/10.3390/cryst9070341
Chicago/Turabian StyleGómez-Morales, Jaime, Luis Antonio González-Ramírez, Cristóbal Verdugo-Escamilla, Raquel Fernández Penas, Francesca Oltolina, Maria Prat, and Giuseppe Falini. 2019. "Induced Nucleation of Biomimetic Nanoapatites on Exfoliated Graphene Biomolecule Flakes by Vapor Diffusion in Microdroplets" Crystals 9, no. 7: 341. https://doi.org/10.3390/cryst9070341
APA StyleGómez-Morales, J., González-Ramírez, L. A., Verdugo-Escamilla, C., Fernández Penas, R., Oltolina, F., Prat, M., & Falini, G. (2019). Induced Nucleation of Biomimetic Nanoapatites on Exfoliated Graphene Biomolecule Flakes by Vapor Diffusion in Microdroplets. Crystals, 9(7), 341. https://doi.org/10.3390/cryst9070341