Rapid Removal and Efficient Recovery of Tetracycline Antibiotics in Aqueous Solution Using Layered Double Hydroxide Components in an In Situ-Adsorption Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of LDH
2.3. Measurement and Characterization
2.4. Removal Procedure
3. Results and Discussion
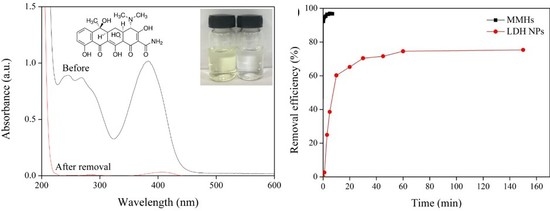
3.1. Removal of TC
3.2. Optimization of the Removal of TC
3.3. Characterization of MMH Sorbent and Confirmation for TC Removal
3.4. Comparison on TC Removal with LDH Sorbent
3.5. Recovery of Captured TC
3.6. Removal of TC in Real Environmental Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, H.; Ma, C.; Song, Y.; Xu, F.; Chen, S.; Wang, L. Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens. Bioelectron. 2013, 50, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Walsh, T.R. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Aminov, R.I.; Krapac, I.J.; Garrigues-Jeanjean, N.; Mackie, R.I. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 2001, 67, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Canizares, P.; Martinez, F.; Jimenez, C.; Lobato, J.; Rodrigo, M. Coagulation and electrocoagulation of wastes polluted with dyes. Environ. Sci. Technol. 2006, 40, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shuang, C.; Liu, F.; Li, A.; Li, Y.; Zhou, Y.; Song, H. Rapid removal of copper with magnetic poly-acrylic weak acid resin: Quantitative role of bead radius on ion exchange. J. Hazard. Mater. 2014, 272, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Peik-See, T.; Pandikumar, A.; Ngee, L.H.; Ming, H.N.; Hua, C.C. Magnetically separable reduced graphene oxide/iron oxide nanocomposite materials for environmental remediation. Catal. Sci. Technol. 2014, 4, 4396–4405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, X.; Liu, X.; Yang, Y.; Pan, F.; Lv, J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Jia, L. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Wu, C.; Xiong, Z.; Lia, C.; Zhang, J. Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution. RSC Adv. 2015, 5, 82127–82137. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Z.; Zhang, C.; Deng, J.; Hou, K.; Cheng, Y.; Zhang, L. Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution. J. Colloid Interface Sci. 2018, 513, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Meng, L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg-Al layered double hydroxide. J. Chem. Eng. Data 2011, 56, 4217–4225. [Google Scholar] [CrossRef]
- Cho, S.; Jung, S.; Jeong, S.; Bang, J.; Park, J.; Park, Y.; Kim, S. Strategy for synthesizing quantum dot-layered double hydroxide nanocomposites and their enhanced photoluminescence and photostability. Langmuir 2013, 29, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Wu, Y.Y.; Liu, C.; Orpe, A.; Liu, Q.; Xu, Z.P.; Qian, G.R.; Qiao, S.Z. Effective self−purification of polynary metal electroplating wastewaters through formation of layered double hydroxides. Environ. Sci. Technol. 2010, 44, 8884–8890. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wu, X.L.; Tan, X.L.; Wang, X.K.; Xu, A.W. One-pot Synthesis of water-swellable Mg−Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As (V) from aqueous solutions. ACS Appl. Mater. Interfaces 2013, 5, 3304–3311. [Google Scholar] [CrossRef] [PubMed]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem. Eng. J. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Rojas, R. Copper, lead and cadmium removal by Ca Al layered double hydroxides. Appl. Clay Sci. 2014, 87, 254–259. [Google Scholar] [CrossRef]
- Goh, K.; Lim, T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Berner, S.; Araya, P.; Govan, J.; Palza, H. Cu/Al and Cu/Cr based layered double hydroxide nanoparticles as adsorption materials for water treatment. J. Ind. Eng. Chem. 2018, 59, 134–140. [Google Scholar] [CrossRef]
- Abdelkader, N.; Bentouami, A.; Derriche, Z.; Bettahar, N.; De Menorval, L.C. Synthesis and characterization of Mg−Fe layer double hydroxides and its application on adsorption of orange G from aqueous solution. Chem. Eng. J. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gasser, M.S. Adsorption study of anionic reactive dye from aqueous solution to Mg−Fe−CO3 layered double hydroxide (LDH). Appl. Surf. Sci. 2012, 259, 650–656. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4125–4155. [Google Scholar] [CrossRef]
- Tian, H.; Bao, W.T.; Jiang, Y.; Wang, L.; Zhang, L.; Sha, O.; Wu, C.; Gao, F. Fabrication of Ni-Al LDH/nitramine-N-doped graphene hybrid composites via a novel self-assembly process for hybrid supercapacitors. Chem. Eng. J. 2018, 354, 1132–1140. [Google Scholar] [CrossRef]
- Liang, T.; Xuan, H.; Xu, Y.; Gao, J.; Han, X.; Yang, J.; Han, P.; Wang, D.; Du, Y. Rational assembly of coal-layered double hydroxide on reduced graphene oxide with enhanced electrochemical performance for energy storage. ChemElectroChem 2018, 5, 2424–2434. [Google Scholar] [CrossRef]
- Xing, J.; Du, J.; Zhang, X.; Shao, Y.; Zhang, T.; Xu, C.A. Ni-P@NiCo LDH core-shell nanorod-decorated nickel foam with enhanced areal specific capacitance for high-performance supercapacitors. Dalton Trans. 2017, 46, 10064–10072. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Ford, W.E.; Szymezak, W.; Schneider, S. The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: A spectroscopic study. J. Phys. Chem. B 1998, 102, 9323–9331. [Google Scholar] [CrossRef]
- Sansuk, S.; Srijaranai, S.; Srijaranai, S. A new approach for removing anionic organic dyes from wastewater based on electrostatically driven assembly. Environ. Sci. Technol. 2016, 50, 6477–6484. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Bao, D.; Zhu, G.; Yang, G.; Geng, J.; Li, H. Effective removal of tetracycline antibiotics from water using hybrid carbon membranes. Sci. Rep. 2017, 7, 43717. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Chen, M.; Zhang, Z.; Xu, J.; Li, D.; Xu, D.; Shi, W. Highly efficient visible-light-driven photocatalytic degradation of tetracycline by a Z-scheme g-C3N4/Bi3TaO7 nanocomposite photocatalyst. Dalton Trans. 2013, 46, 8431–8438. [Google Scholar] [CrossRef]
- Penneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, Bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Zyoud, A.; Jondi, W.; AlDaqqah, N.; Asaad, S.; Qamhieh, N.; Hajamohideen, A.; Helal, M.; Kwon, H.; Hilal, H. Self-sensitization of tetracycline degradation with simulated solar light catalyzed by ZnO@montmorillonite. Solid State Sci. 2017, 74, 131–143. [Google Scholar] [CrossRef]








| Material | Removal Method | Removal Time (min) | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| GO/AC hybrid membrane | filtration | 19 | 98.9 | [28] |
| g-C3N4/Bi3TaO7 | photocatalytic | 90 | 89.2 | [29] |
| Carbon doped g-C3N4 | photocatalytic | 90 | 90 | [30] |
| ZnO@montmorillonite | photocatalytic | 75 | 94 | [31] |
| MWCNTs | adsorption | 20 | 99.8 | [8] |
| GO-MPs | adsorption | 10 | 98 | [9] |
| Fe/Ni BNPs | adsorption | 120 | 97.4 | [11] |
| MMHs | in situ-adsorption | 4 | 99.8 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panplado, K.; Subsadsana, M.; Srijaranai, S.; Sansuk, S. Rapid Removal and Efficient Recovery of Tetracycline Antibiotics in Aqueous Solution Using Layered Double Hydroxide Components in an In Situ-Adsorption Process. Crystals 2019, 9, 342. https://doi.org/10.3390/cryst9070342
Panplado K, Subsadsana M, Srijaranai S, Sansuk S. Rapid Removal and Efficient Recovery of Tetracycline Antibiotics in Aqueous Solution Using Layered Double Hydroxide Components in an In Situ-Adsorption Process. Crystals. 2019; 9(7):342. https://doi.org/10.3390/cryst9070342
Chicago/Turabian StylePanplado, Kwanjira, Maliwan Subsadsana, Supalax Srijaranai, and Sira Sansuk. 2019. "Rapid Removal and Efficient Recovery of Tetracycline Antibiotics in Aqueous Solution Using Layered Double Hydroxide Components in an In Situ-Adsorption Process" Crystals 9, no. 7: 342. https://doi.org/10.3390/cryst9070342
APA StylePanplado, K., Subsadsana, M., Srijaranai, S., & Sansuk, S. (2019). Rapid Removal and Efficient Recovery of Tetracycline Antibiotics in Aqueous Solution Using Layered Double Hydroxide Components in an In Situ-Adsorption Process. Crystals, 9(7), 342. https://doi.org/10.3390/cryst9070342