Polarization Dependence of Low-Frequency Vibrations from Multiple Faces in an Organic Single Crystal
Abstract
:1. Introduction
2. Materials and Methods
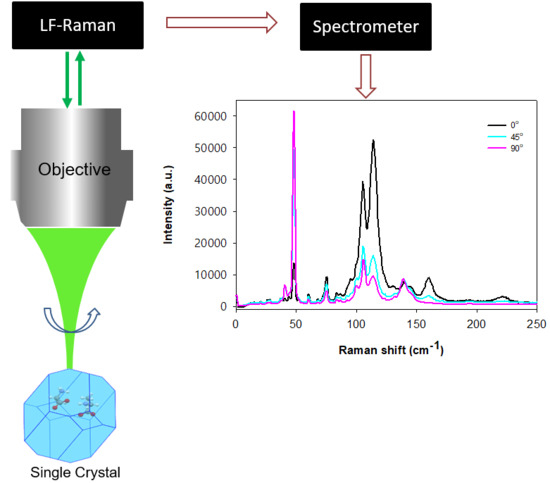
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Gues-Hornedo, N.R. Denette Murphy Significance of Controlling Crystallization Mechanisms and Kinetics in Pharmaceutical Systems. J. Pharm. Sci. 1999, 88, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, I.; Arbiol, J.; Dezanneau, G.; Cornet, A.; Morante, J.R. Crystalline structure, defects and gas sensor response to NO2 and H2S of tungsten trioxide nanopowders. Sens. Actuators B Chem. 2003, 93, 475–485. [Google Scholar] [CrossRef]
- Tabi, T.; Sajó, I.E.; Szabó, F.; Luyt, A.S.; Kovács, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Aviv, H.; Nemtsov, I.; Mastai, Y.; Tischler, Y.R. Characterization of Crystal Chirality in Amino Acids Using Low-Frequency Raman Spectroscopy. J. Phys. Chem. A 2017, 121, 7882–7888. [Google Scholar] [CrossRef] [PubMed]
- Nemtsov, I.; Mastai, Y.; Tischler, Y.R.; Aviv, H. Chiral Purity of Crystals Using Low-Frequency Raman Spectroscopy. ChemPhysChem 2018, 19, 3116–3121. [Google Scholar] [CrossRef]
- Crasto, D.; Malola, S.; Brosofsky, G.; Dass, A.; Hakkinen, H. Single crystal XRD structure and theoretical analysis of the chiral Au30S (S-t-Bu) 18 cluster. J. Am. Chem. Soc. 2014, 136, 5000–5005. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 μm in solution grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–971. [Google Scholar] [CrossRef]
- Bianconi, A. Surface X-Ray absorption spectroscopy: surface EXAFS and surface XANES. Appl. Surf. Sci. 1980, 6, 392–418. [Google Scholar] [CrossRef]
- Yano, J.; Yachandra, V.K. X-ray absorption spectroscopy. Photosynth. Res. 2009, 102, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiff, D.; Aviv, H.; Rosenbaum, E.; Tischler, Y.R. Spectroscopic Method for Fast and Accurate Group A Streptococcus Bacteria Detection. Anal. Chem. 2016, 88, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Aviv, H.; Berezin, S.; Agai, O.; Sinwani, M.; Tischler, Y.R. Deposition and Characterization of Roughened Surfaces. Langmuir 2017, 33, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.C.; Aschaffenburg, D.J.; Ofori-Okai, B.K.; Schmuttenmaer, C.A. Intermolecular vibrations in hydrophobic amino acid crystals: Experiments and calculations. J. Phys. Chem. B 2013, 117, 10444–10461. [Google Scholar] [CrossRef] [PubMed]
- Niehues, G.; Heyden, M.; Schmidt, D.A.; Havenith, M.; Berzofsky, J.A.; DeLisi, C.; Haller, E.E.; Havenith, M. Exploring hydrophobicity by THz absorption spectroscopy of solvated amino acids. Faraday Discuss. 2011, 150, 193–207. [Google Scholar] [CrossRef]
- Kalanoor, B.S.; Ronen, M.; Oren, Z.; Gerber, D.; Tischler, Y.R. New Method to Study the Vibrational Modes of Biomolecules in the Terahertz Range Based on a Single-Stage Raman Spectrometer. ACS Omega 2017, 2, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Woods, K.N.; Pfeffer, J.; Dutta, A.; Klein-Seetharaman, J.; Haak, J.R. Vibrational resonance, allostery, and activation in rhodopsin-like G protein-coupled receptors. Sci. Rep. 2016, 6, 37290–37306. [Google Scholar] [CrossRef]
- Takahashi, M.; Okamura, N.; Ding, X.; Shirakawa, H.; Minamide, H. Intermolecular hydrogen bond stretching vibrations observed in terahertz spectra of crystalline vitamins. CrystEngComm 2018, 20, 1960–1969. [Google Scholar] [CrossRef]
- Hariharan, P.; Oreb, B.F.; Brown, N. A digital phase-measurement system for real-time holographic interferometry. Opt. Commun. 1982, 41, 393–396. [Google Scholar] [CrossRef]
- Marguí, E.; Van Meel, K.; Van Grieken, R.; Buendía, A.; Fontàs, C.; Hidalgo, M.; Queralt, I. Method for the determination of Pd-catalyst residues in active pharmaceutical ingredients by means of high-energy polarized-beam energy dispersive X-ray fluorescence. Anal. Chem. 2009, 81, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Milner, T.E.; van Gemert, M.J.C.; Nelson, J.S. Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography. Opt. Lett. 1997, 22, 934–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-C.; Kim, E.-S. A novel configuration of LCD projectors for efficient orthogonal polarization of two projected views. Opt. Commun. 2006, 266, 55–66. [Google Scholar] [CrossRef]
- Iizuka, K. Welcome to the wonderful world of 3D: introduction, principles and history. Opt. Photonics News 2006, 17, 42–51. [Google Scholar] [CrossRef]
- Padgett, M.; Allen, L. Light with a twist in its tail. Contemp. Phys. 2000, 41, 275–285. [Google Scholar] [CrossRef]
- Duesberg, G.S.; Loa, I.; Burghard, M.; Syassen, K.; Roth, S. polarized Raman. Phys. Rev. Lett. 2000, 85, 5436–5439. [Google Scholar] [CrossRef]
- Haesler, J.; Schindelholz, I.; Riguet, E.; Bochet, C.G.; Hug, W. Absolute configuration of chirally deuterated neopentane. Nature 2007, 446, 526. [Google Scholar] [CrossRef]
- Barron, L.D.; Zhu, F.; Hecht, L.; Tranter, G.E.; Isaacs, N.W. Raman optical activity: An incisive probe of molecular chirality and biomolecular structure. J. Mol. Struct. 2007, 834, 7–16. [Google Scholar] [CrossRef]
- Gorelik, V.S.; Kaminskii, A.A.; Melnik, N.N.; Sverbil, P.P.; Voinov, Y.P.; Zavaritskaya, T.N.; Zlobina, L.I. Spontaneous Raman scattering spectra of ADP and DADP crystals in different polarization schemes. J. Russ. Laser Res. 2008, 29, 357–363. [Google Scholar] [CrossRef]
- Maeda, Y.; Udono, H.; Terai, Y. Raman spectra for β-FeSi2 bulk crystals. Thin Solid Films 2004, 461, 165–170. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.U.; Lee, J.; Park, H.J.; Lee, Z.; Lee, C.; Cheong, H. Anomalous polarization dependence of Raman scattering and crystallographic orientation of black phosphorus. Nanoscale 2015, 7, 18708–18715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Storms, R.D. Raman Study of Hydrogen Bonding and Long-Wavelength Lattice Modes in an L-Alanine Single Crystal. J. Chem. Phys. 1971, 55, 5110–5119. [Google Scholar] [CrossRef]
- Razzetti, C.; Ardoino, M.; Zanotti, L.; Zha, M.; Paorici, C. Solution growth and characterisation of L-alanine single crystals. Cryst. Res. Technol. 2002, 37, 456–465. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, X.; Li, H.; Zhang, J.; Araujo, P.T.; Gan, C.K.; Wu, J.; Zhang, H.; Quek, S.Y.; Dresselhaus, M.S.; et al. Interlayer breathing and shear modes in few-trilayer MoS2 and WSe2. Nano Lett. 2013, 13, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Men, Z.; Li, S.; Wang, S.; Li, Z.; Sun, C. Study of hydrogen bonding in ethanol-water binary solutions by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 621–624. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemtsov, I.; Aviv, H.; Mastai, Y.; Tischler, Y.R. Polarization Dependence of Low-Frequency Vibrations from Multiple Faces in an Organic Single Crystal. Crystals 2019, 9, 425. https://doi.org/10.3390/cryst9080425
Nemtsov I, Aviv H, Mastai Y, Tischler YR. Polarization Dependence of Low-Frequency Vibrations from Multiple Faces in an Organic Single Crystal. Crystals. 2019; 9(8):425. https://doi.org/10.3390/cryst9080425
Chicago/Turabian StyleNemtsov, Irena, Hagit Aviv, Yitzhak Mastai, and Yaakov R. Tischler. 2019. "Polarization Dependence of Low-Frequency Vibrations from Multiple Faces in an Organic Single Crystal" Crystals 9, no. 8: 425. https://doi.org/10.3390/cryst9080425
APA StyleNemtsov, I., Aviv, H., Mastai, Y., & Tischler, Y. R. (2019). Polarization Dependence of Low-Frequency Vibrations from Multiple Faces in an Organic Single Crystal. Crystals, 9(8), 425. https://doi.org/10.3390/cryst9080425