Press to Success: Gd5FW3O16—The First Gadolinium(III) Fluoride Oxidotungstate(VI)
Abstract
:1. Introduction
2. Materials and Methods
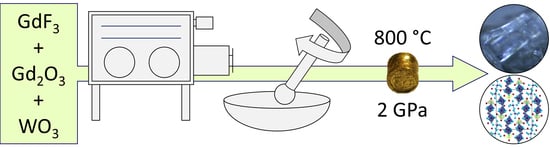
2.1. High-Pressure Synthesis
2.2. Single-Crystal Structure Determination
2.3. Single-Crystal Raman Spectroscopy
3. Results and Discussion
3.1. Crystal Structure
3.2. Single-Crystal Raman Spectrum
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Andac, O.; Glasser, F.P.; Howie, R.A. Dicalcium Sodium Fluoride Silicate. Acta Crystallogr. 1997, C 53, 831–833. [Google Scholar] [CrossRef]
- Hartenbach, I.; Schleid, Th. Na3F[WO4]: A Sodium Fluoride Ortho-Oxotungstate(VI) with Strands of Face-Shared Fluoride-Centred Sodium Octahedra According to {[FNa6/2]2+}. Z. Anorg. Allg. Chem. 2007, 633, 524–526. [Google Scholar] [CrossRef]
- Moutou, J.M.; Vlasse, M.; Cervera-Marzal, M.; Chaminade, J.P.; Pouchard, M.A. Structural Study of a New Lithium Oxyfluorotungstate, LiW3O9F. J. Solid State Chem. 1984, 51, 190–195. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P.; Ravez, J. Structural Study of a New Lithium Oxyfluorotungstate, LiW3O9F. Acta Crystallogr. 1989, B 45, 364–370. [Google Scholar] [CrossRef]
- Hoard, J.L.; Vincent, W.B. Structures of Complex Fluorides. Potassium Hexafluogermanate and Ammonium Hexafluogermanate. J. Am. Chem. Soc. 1939, 61, 2849–2852. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Ackerman, J.F. Structure and Luminescence of Cs2WO2F4: Efficient Luminescence of Isolated (WO2F4)2− Octahedra. J. Solid State Chem. 1992, 98, 144–150. [Google Scholar] [CrossRef]
- Udovenko, A.A.; Laptash, N.M. Disorder in Crystals of Dioxofluorotungstates, (NH4)2WO2F4 and Rb2WO2F4. Acta Crystallogr. 2008, B 64, 645–651. [Google Scholar] [CrossRef]
- Mazej, Z.; Gilewski, T.; Goreshnik, E.A.; Jaglicic, Z.; Derzsi, M.; Grochala, W. Canted Antiferromagnetism in Two-Dimensional Silver(II) Bis[Pentafluoridooxidotungstate(VI)]. Inorg. Chem. 2017, 56, 224–233. [Google Scholar] [CrossRef]
- Moutou, J.M.; Francisco, R.H.P.; Chaminade, J.P.; Pouchard, M.; Hagenmuller, P. Characterisation Structurale de l’Oxyflurotungstate de Cuivre CuWO3F2. Z. Anorg. Allg. Chem. 1986, 539, 165–174. [Google Scholar] [CrossRef]
- Wingefeld, G.; Hoppe, R. Zur Konstitution von Ba2WO3F4 und Ba2MoO3F4. Z. Anorg. Allg. Chem. 1984, 518, 149–160. [Google Scholar] [CrossRef]
- Torardi, C.C.; Brixner, L.H. Structure and Luminescence of Ba2WO3F4. Mater. Res. Bull. 1984, 20, 137–145. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P.; Ravez, J. Ferroelectric Structure and Related Properties of Pb5W3O9F10. J. Chem. Phys. 1987, 87, 6012–6020. [Google Scholar] [CrossRef]
- Schleid, Th.; Strobel, S.; Dorhout, P.K.; Nockemann, P.; Binnemans, K.; Hartenbach, I. YF[MoO4] and YCl[MoO4]: Two Halide Derivatives of Yttrium Ortho-Oxomolybdate-Syntheses, Structures, and Luminescence Properties. Inorg. Chem. 2008, 47, 3728–3735. [Google Scholar] [CrossRef] [PubMed]
- Schustereit, T.; Schleid, Th.; Hartenbach, I. Solvochemical Synthesis and Crystal Structure of the Fluoride-Derivatized Early Lanthanoid(III) ortho-Oxidomolybdates(VI) LnF[MoO4] (Ln = Ce − Nd). Eur. J. Inorg. Chem. 2014, 2014, 5145–5151. [Google Scholar] [CrossRef]
- Hartenbach, I.; Strobel, S.; Dorhout, P.K.; Schleid, Th. Crystal Structure, Spectroscopic Properties, and Magnetic Behaviour of the Fluoride-Derivatized Lanthanoid(III) Ortho-Oxomolybdates(VI) LnF[MoO4] (Ln = Sm − Tm). J. Solid State Chem. 2008, 181, 2828–2836. [Google Scholar] [CrossRef]
- Hartenbach, I.; Höppe, H.A.; Kazmierczak, K.; Schleid, Th. Synthesis, Crystal Structure and Spectroscopic Properties of a Novel Yttrium(III) Fluoride Dimolybdate(VI): YFMo2O7. Dalton Trans. 2014, 43, 14016–14021. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.L.; Blaschkowski, B.; Hartenbach, I. No Pyroanions: The Representatives of the Lanthanoid(III) Fluoride Oxidodimolybdates(VI) LnFMo2O7 with the Smaller Cations (Ln = Eu − Yb). J. Solid State Chem. 2017, 251, 237–242. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) Handbook of Chemistry and Physics, Internet Version, 98 ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2019. [Google Scholar]
- Perets, S.; Tseitlin, M.; Shneck, R.Z.; Mogiliyanski, D.; Kimmel, G.; Burshtein, Z. Sodium Gadolinium Tungstate NaGd(WO4)2: Growth, Crystallography, and Some Physical Properties. J. Cryst. Growth 2007, 305, 257–264. [Google Scholar] [CrossRef]
- Naruke, H.; Yamase, T. Structures of Novel R2Mo5O18 and R6Mo12O45 (R = Eu and Gd) prepared by thermal decomposition of polyoxomolybdate precursor (R2(H2O)12Mo8O27·nH2O). Inorg. Chem. 2002, 41, 6514–6520. [Google Scholar] [CrossRef]
- Schustereit, T. Synthese und Charakterisierung anionischer Derivate von Seltenerdmetall-Oxidomolybdaten und -wolframaten mit Hilfe festkörper- und nasschemischer Methoden. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Dorn, K.V. Synthese und Charakterisierung anionischer sowie lithiumhaltiger Derivate von Seltenerdmetall(III)-Oxidomolybdaten(VI) und -wolframaten(VI). Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2018. [Google Scholar]
- Dorn, K.V.; Blaschkowski, B.; Förg, K.; Netzsch, P.; Höppe, H.A.; Hartenbach, I. Prism Inside: Spectroscopic and Magnetic Properties of the Lanthanide(III) Chloride Oxidotungstates(VI) Ln3Cl3[WO6] (Ln = La − Nd, Sm − Tb). Z. Anorg. Allg. Chem. 2017, 643, 1642–1648. [Google Scholar] [CrossRef]
- Hartenbach, I.; Strobel, S.; Schleid, Th.; Sarkar, B.; Kaim, W.; Nockemann, P.; Binnemans, K.; Dorhout, P.K. Synthesis, Structure, and Spectroscopic Properties of the New Lanthanum(III) Fluoride Oxomolybdate(VI) La3FMo4O16. Eur. J. Inorg. Chem. 2010, 2010, 1626–1632. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C 71, 3–8. [Google Scholar]
- Hahn, Th.; Wilson, A.J.C. International Tables for Crystallography, 2nd ed.; Kluwer Academic Publishers: Boston, MA, USA, 1992. [Google Scholar]
- Fischer, R.X.; Tillmanns, E. The Equivalent Isotropic Displacement Factor. Acta Crystallogr. 1988, C 44, 775–776. [Google Scholar] [CrossRef]
- Hoppe, R. Madelung Constants as a new Guide in the Structural Chemistry of Solids. Adv. Fluorine Chem. 1970, 6, 387–438. [Google Scholar]
- Hoppe, R. The Madelung Part of Lattice Energy, MAPLE, as a Guide in the Structural Solid State Chemistry. Izv. Jugoslav. Centr. Krist. (Zagreb) 1973, 8, 21–36. [Google Scholar]
- Hoppe, R. The Madelung Part of Lattice Energy, MAPLE, as a Guide in Crystal Chemistry. In Crystal Structure and Chemical Bonding in Inorganic Chemistry; Rooymans, C.J.M., Rabenau, A., Eds.; North-Holland Publishers: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Hoppe, R. Effective Coordination Numbers (ECoN) and Mean Fictive Ionic Radii (MEFIR). Z. Kristallogr. 1979, 150, 23–52. [Google Scholar] [CrossRef]
- Grzyb, T.; Wiglusz, R.J.; Nagirnyi, V.; Kotlov, A.; Lis, S. Revised crystal structure and luminescent properties of gadolinium oxyfluoride Gd4O3F6 doped with Eu3+ ions. Dalton Trans. 2014, 43, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B 41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Crystallogr. 1991, B 47, 192–197. [Google Scholar] [CrossRef]
- Spirlet, M.-R.; Busing, W.R. Dodecatungstophosphoric acid-21-water by neutron diffraction. Acta Crystallogr. 1977, B 34, 907–910. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter Publisher: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Busca, G. Differentiation of Monooxo and Polyoxo and of Monomeric and Polymeric Vanadate, Molybdate and Tungstate Species in Metal Oxide Catalysts by IR and Raman Spectroscopy. J. Raman Spectrosc. 2002, 33, 348–358. [Google Scholar] [CrossRef]
- Weidlein, J.; Müller, U.; Dehnicke, K. Schwingungsfrequenzen I-Hauptgruppenelemente, 1st ed.; Georg Thieme Verlag: Stuttgart, Germany, 1981. [Google Scholar]






| Crystal System | Monoclinic |
|---|---|
| Space group | P21/c (no. 14) |
| Formula units, Z | 4 |
| a/pm | 539.29(4) |
| b/pm | 1556.41(12) |
| c/pm | 1522.66(11) |
| β/° | 93.452(4) |
| Density, Dx/g·cm−3 | 8.397 |
| Molar volume, V/cm3·mol−1 | 192.07 |
| Single-crystal X-ray diffractometer | Smart Apex II Duo (Bruker AXS) |
| Radiation | 71.07 (Mo-Kα) |
| Temperature, T/K | 130(2) |
| Data corrections | Program SADABS [25] |
| Structure solution and refinement | Program package SHELX-2013 [26], scattering factors according to [27] |
| Index range, ±h/±k/±l | 8/23/23 |
| 2θmax/° | 66.40 |
| F (000) | 2716 |
| Absorption coefficient, μ | 52.64 |
| Reflections collected/unique | 22613/4758 |
| Refined parameters | 147 |
| Rint/Rσ | 0.058/0.059 |
| R1 for (n) reflections with |Fo| ≥ 4σ (Fo) | 0.033 (3728) |
| R1/wR2 for all reflections | 0.052/0.047 |
| Goodness of Fit, S | 1.005 |
| Extinction, g | 0.00078 (1) |
| Residual electron density, ρmin/max/e− 10−6 pm−3 | −4.98/3.95 at (Gd5)3+ |
| Atom | Position | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|---|
| Gd1 | 4e | 0.54505(6) | 0.22505(2) | 0.86095(2) | 51.2(8) |
| Gd2 | 4e | 0.99526(6) | 0.15440(2) | 0.22655(2) | 47.8(7) |
| Gd3 | 4e | 0.02811(6) | 0.11780(2) | 0.47811(2) | 48.0(8) |
| Gd4 | 4e | 0.48355(6) | 0.02332(2) | 0.12010(2) | 43.4(8) |
| Gd5 | 4e | 0.98901(8) | 0.09537(3) | 0.72614(3) | 152.9(10) |
| F | 4e | 0.1920(7) | 0.1929(3) | 0.3634(3) | 67(9) |
| W1 | 4e | 0.00855(5) | 0.10733(2) | 0.97043(2) | 41.7(7) |
| W2 | 4e | 0.54455(5) | 0.21721(2) | 0.63497(2) | 42.4(6) |
| W3 | 4e | 0.56511(5) | 0.02705(2) | 0.34505(2) | 42.7(6) |
| O1 | 4e | 0.8804(8) | 0.2125(3) | 0.9665(3) | 58(10) |
| O2 | 4e | 0.1430(9) | 0.1177(3) | 0.0836(3) | 67(10) |
| O3 | 4e | 0.2830(9) | 0.1171(3) | 0.9023(3) | 67(11) |
| O4 | 4e | 0.7770(8) | 0.0232(3) | 0.0125(3) | 48(10) |
| O5 | 4e | 0.8007(8) | 0.0847(3) | 0.8614(3) | 50(10) |
| O6 | 4e | 0.3607(9) | 0.2020(3) | 0.5350(3) | 80(11) |
| O7 | 4e | 0.6309(9) | 0.1708(3) | 0.1260(3) | 57(10) |
| O8 | 4e | 0.8288(9) | 0.1619(3) | 0.5964(3) | 75(11) |
| O9 | 4e | 0.2937(9) | 0.2284(3) | 0.7170(3) | 85(11) |
| O10 | 4e | 0.7822(8) | 0.2219(3) | 0.7491(3) | 46(10) |
| O11 | 4e | 0.5031(9) | 0.0898(3) | 0.6852(3) | 98(11) |
| O12 | 4e | 0.3689(9) | 0.0384(3) | 0.4321(3) | 80(10) |
| O13 | 4e | 0.3196(9) | 0.0591(3) | 0.2558(3) | 101(11) |
| O14 | 4e | 0.7181(9) | 0.1391(3) | 0.3603(3) | 83(11) |
| O15 | 4e | 0.1307(9) | 0.0152(3) | 0.5929(3) | 64(10) |
| O16 | 4e | 0.7875(8) | 0.0239(3) | 0.2326(3) | 51(10) |
| Distance | Distance | Distance | Distance | ||||
|---|---|---|---|---|---|---|---|
| Gd1–F | 229.4(4) | Gd2–F | 235.8(4) | Gd3–F | 232.1(4) | Gd4–O2 | 239.1(5) |
| Gd1–O1 | 235.4(5) | Gd2–O2 | 243.0(5) | Gd3–O1 | 276.1(5) | Gd4–O3 | 255.5(5) |
| Gd1–O3 | 230.8(5) | Gd2–O7 | 243.1(5) | Gd3–O6 | 234.4(5) | Gd4–O4 | 234.5(5) |
| Gd1–O5 | 258.3(5) | Gd2–O9 | 244.3(5) | Gd3–O8 | 225.9(5) | Gd4–O4’ | 249.7(5) |
| Gd1–O9 | 250.8(5) | Gd2–O10 | 227.9(5) | Gd3–O12 | 235.6(5) | Gd4–O5 | 230.3(5) |
| Gd1–O10 | 219.0(5) | Gd2–O13 | 231.6(5) | Gd3–O14 | 240.0(5) | Gd4–O7 | 242.9(5) |
| Gd1–O14 | 231.2(5) | Gd2–O14 | 260.9(5) | Gd3–O15 | 240.8(5) | Gd4–O13 | 236.6(5) |
| Gd2–O16 | 232.4(5) | Gd3–O15’ | 246.5(5) | Gd4–O16 | 229.8(5) | ||
| Gd5–O5 | 235.7(5) | W1–O1 | 177.6(5) | W2–O6 | 178.2(5) | W3–O11 | 190.6(5) |
| Gd5–O8 | 234.8(5) | W1–O2 | 183.6(5) | W2–O7 | 181.1(5) | W3–O12 | 175.4(5) |
| Gd5–O9 | 265.2(5) | W1–O3 | 186.4(5) | W2–O8 | 188.3(5) | W3–O13 | 190.5(5) |
| Gd5–O10 | 230.0(5) | W1–O4 | 194.5(5) | W2–O9 | 190.4(5) | W3–O14 | 193.7(5) |
| Gd5–O11 | 265.8(5) | W1–O4’ | 234.4(5) | W2–O10 | 209.8(5) | W3–O15 | 195.7(5) |
| Gd5–O11’ | 287.9(5) | W1–O5 | 197.8(5) | W2–O11 | 214.3(5) | W3–O16 | 215.0(5) |
| Gd5–O13 | 294.6(5) | ||||||
| Gd5–O15 | 253.9(5) | ||||||
| Gd5–O16 | 228.1(5) |
| Angle | ||
|---|---|---|
| Gd1–F–Gd2 | (1x) | 117.2(2) |
| Gd1–F–Gd3 | (1x) | 130.3(2) |
| Gd2–F–Gd3 | (1x) | 111.4(2) |
| W1–O4–W1 | (2x) | 107.3(2) |
| O4–W1–O4’ | (2x) | 72.7(2) |
| W2–O11–W3 | (1x) | 145.0(3) |
| Atom | Gd1 | Gd2 | Gd3 | Gd4 | Gd5 | W1 | W2 | W3 | C.N. |
|---|---|---|---|---|---|---|---|---|---|
| F | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3 |
| O1 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 3 |
| O2 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 3 |
| O3 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 3 |
| O4 | 0/0 | 0/0 | 0/0 | 2/2 | 0/0 | 2/2 | 0/0 | 0/0 | 4 |
| O5 | 1/1 | 0/0 | 0/0 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 4 |
| O6 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 2 |
| O7 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 3 |
| O8 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 3 |
| O9 | 1/1 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 4 |
| O10 | 1/1 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 4 |
| O11 | 0/0 | 0/0 | 0/0 | 0/0 | 2/2 | 0/0 | 1/1 | 1/1 | 4 |
| O12 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 2 |
| O13 | 0/0 | 1/1 | 0/0 | 1/1 | 1/1 | 0/0 | 0/0 | 1/1 | 4 |
| O14 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 4 |
| O15 | 0/0 | 0/0 | 2/2 | 0/0 | 1/1 | 0/0 | 0/0 | 1/1 | 4 |
| O16 | 0/0 | 1/1 | 0/0 | 1/1 | 1/1 | 0/0 | 0/0 | 1/1 | 4 |
| C.N. | 7 | 8 | 8 | 8 | 9 | 6 | 6 | 6 | |
| ECoN | 6.2 | 7.4 | 7.2 | 7.6 | 6.3 | 4.8 | 4.8 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorn, K.V.; Hartenbach, I. Press to Success: Gd5FW3O16—The First Gadolinium(III) Fluoride Oxidotungstate(VI). Crystals 2019, 9, 424. https://doi.org/10.3390/cryst9080424
Dorn KV, Hartenbach I. Press to Success: Gd5FW3O16—The First Gadolinium(III) Fluoride Oxidotungstate(VI). Crystals. 2019; 9(8):424. https://doi.org/10.3390/cryst9080424
Chicago/Turabian StyleDorn, Katharina V., and Ingo Hartenbach. 2019. "Press to Success: Gd5FW3O16—The First Gadolinium(III) Fluoride Oxidotungstate(VI)" Crystals 9, no. 8: 424. https://doi.org/10.3390/cryst9080424
APA StyleDorn, K. V., & Hartenbach, I. (2019). Press to Success: Gd5FW3O16—The First Gadolinium(III) Fluoride Oxidotungstate(VI). Crystals, 9(8), 424. https://doi.org/10.3390/cryst9080424