1. Introduction
Optoelectronic devices, widely used in the semiconductor industry, are sensitive to environmental conditions, and therefore their operation is compromised. In order to mitigate this, an effective protection of the semiconductor materials against environmental conditions is necessary. The permeation of the water molecules into the device structure induces degradation of both the electrodes and the semiconductor itself. Metallophthalocyanines (MPcs), as organic semiconductors, have shown outstanding electronic and optical properties for optoelectronic materials, which is a consequence of their electronic delocalization [
1,
2,
3,
4,
5,
6]. These properties include, as well, photosensitivity, flexible structural modification and strong absorbance in the 600–800 nm region [
4,
7,
8]. Also, it is known that the bandgap energy between HOMO (Highest Occupied Molecular) and LUMO (Lowest Unoccupied Molecular Orbital) can be tuned for these materials, as well as for photoluminescence (PL) and light absorption [
5,
6,
9]. MPcs, formed by certain type of substituents or functional groups in their structure, present great chemical stability and are insoluble in most solvents [
10], but not in water [
10,
11,
12]. For this reason, it is important to protect the MPc without affecting the charge transport property in this type of semiconductor. The use of polymeric membranes is an option that may solve the requirements of the semiconductor isolation against the environmental water. Polymer Nylon 11, also known as Rilsan
©, is a polyamide that combines qualities of flexibility, high resistance to aging, and excellent impact resistance (even at high temperatures) in high water or humid environments [
13,
14,
15]. These materials present a high melting point and relatively low flexural modulus and hardness compared to different thermoplastic polymers that make them good candidates for polymeric membranes applications, which result from the amide linkages in the chain. Additionally, there are other polymers with similar mechanical properties like polypropylene, polymethylpentene, ethylene tetrafluoroethylene, and polytetrafluoro ethylene [
13]. These polymers do not present some of the three requirements needed for a suitable protection of the MPcs for optoelectronic devices: (i) chemical resistance to organic solvents and environmental agents like humidity, (ii) ability to form uniform thin films, and (iii) effective integration between the polymeric matrix and the MPc semiconductor particles. Poly(octyl methacrylate-
co-vinylimidazole [
10], polystyrene, polyacrylonitrile [
11], and poly(arylene ether sulfone) [
16] have been used in the manufacture of membranes along with MPcs; however, these membrane applications were not intended for optoelectronic devices. Therefore, the Nylon 11 offers a viable option for the manufacture of semiconductor membranes as described in our previous studies [
17]. The amide linkages in the chain provide these properties, among others, through the hydrogen bonds, allowing a solid interaction between the chains. Also, molecules that create hydrogen bonds can penetrate and interfere in the inter-chain hydrogen bonds weakening the hydrogen bond network [
17]. Due to Nylon low permeability it is frequently used as a bagging material during shipping or storage. The mechanism by which a material in a gaseous state passes through a solid material of cross sectional area
A and thickness
d and is related to a pressure difference among it is called permeation [
18,
19,
20]. Thomas Graham measured the permeability intensity of some gases. He was the first one who described a solution-permeation model for polymeric membranes, and his research on porous membranes led him to record Graham’s Law of Effusion [
21]. It has been observed by Schowalter et al. that Nylon has lower permeability for many gases in comparison to similar materials [
18]. An exponential dependence of the permeation of some polymers upon the square of the permeating gas atomic radius has been reported [
19,
20,
22,
23]. Electrospun fibres, as a support material, have been used by Mafukidze et al. and resulted in a lack of mechanical strength, which polyamides membranes employed in this work may possess [
11]. Therefore, introducing MPcs as molecular materials whiting a polymer, such as Nylon 11, could promote outstanding and effective properties in membrane applications and cause them to remain intact in the fiber [
11,
24,
25,
26,
27,
28,
29]. We report in this communication the fabrication of semiconductor membranes consisting of MgPc-allenes particles dispersed in Nylon 11 films. These membranes combine polymers properties with organic semiconductor properties and also provide a barrier effect for the permeating water molecules. We describe the fabrication and characterization of the obtained membranes by Fourier-transform infrared spectroscopy (FT-IR) spectroscopy, scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS), UV-ViS optical absorption measurements, Photoluminescence (PL), null Ellipsometry, and electrical Current-Voltage (
I-V) characteristics using four point probes that were used to determine the chemical, microstructural, and optoelectronic properties. A further study of the semiconductor membranes should be done in order to determine their performance under harmful environmental conditions and the degradation relation of their optoelectronic properties such as bandgap and conductivity, even though the semiconductor membranes’ photo-degradation was analyzed by accelerated conditions during 8 h of light exposition.
3. Results and Discussion
Due to drastic temperature changes during the deposition and the thermal relaxation that implies the manufacture of the membranes, the IR spectroscopy was carried out for intrinsic, doped MgPc thin films, and finally, for the Nylon 11 membrane (
Table 1). With the aim of monitoring any chemical change in the material and beside the modifications in the crystalline structure of semiconductor, IR spectroscopy was made. The spectra for the MgPc include a band around 1610 cm
−1 corresponding to C–C stretching vibrations within the macrocyclic ring. The band around 1333 cm
−1 is assigned to the C=N vibrations in the macrocyclic ring, with around 1285 and 756 cm
−1 assigned to the isoindole plane and C–N stretching vibrations, respectively, and around 1421, 1163, and 1117 cm
−1 resulting from the interaction of carbon with the peripheral ring hydrogen atoms. On the other hand, the value around 3080 cm
−1 corresponds to the vibration of the O–H bond of the carboxylic acid C(O)O–H in the allene, and around 2926 cm
−1 likewise corresponds to the vibration related to the C–H bonds of the methoxy substituents. Finally, the bands around 3308 and 3077 cm
−1 correspond to the stretching vibrations for N–H, and the band around 1652 cm
−1 corresponds to the C=O vibrations of the polymeric matrix. The crystalline structure of the MgPc is another variant that can be monitored by IR spectroscopy, due to its different polymorphs forms [
33,
34,
35,
36,
37]. The MgPc α-form and the β-form can be identified by a band around 720 cm
−1 and a band around 778 cm
−1, respectively [
33,
34,
35,
36,
37]. The MgPc keeps its two crystalline structures α and β during the fabrication process of the membrane (see
Table 1). Apparently by surrounding the semiconductor particles, the Nylon 11 inhibits the phase change, and, according to what is observed in
Table 1, the doped MgPc did not suffer any chemical changes during the fabrication of the membrane. It is important to consider that, according to Jonathan Albo et al., polyamide membranes may be affected by the presence of gaseous molecules (water, gases, or solvents) [
38,
39]. The gas molecules’ size, polarity, and type of interactions generate these effects. The latter is the reason why, according to the application of the membrane, the polymer may need a pretreatment [
38,
39]. This pretreatment may include the cleaning of the membrane by solvents and the drying of the residual solvent that could be inside the structure of the polymer. A drying pretreatment at different temperatures can also be carried out followed by a drying process with solvents [
38]. Considering the previous factors, in the present study the membrane was prepared by the physical method of Nylon11 sublimation at high vacuum. This process implies the heating of the polymer at 573 K, which generates the separation from the polymer of the water molecules and subsequently the elimination by the vacuum of the chamber. Additionally, the thermal process for the incorporation of MPc into Nylon 11 is carried out at 413 K; this temperature favors the elimination of water molecules that can remain within the membrane, and it is confirmed by the IR spectrum [
40]. Furthermore, the sublimation at high vacuum and thermal relaxation of Nylon 11 was selected, unlike other membrane manufacturing processes, in order to avoid the presence of solvents that can interact with the polymer [
10,
11,
12]. Therefore, we may conclude that the manufacturing process of the membrane from doped MgPc-Nylon 11 by high vacuum evaporation, and later by thermal relaxation, is appropriate.
With respect to SEM Micrographs shown in
Figure 2a, the membrane is observed to be formed by particles of agglomerated (most of them), with rounded structure of different sizes. During the deposition of the membrane on the substrate, the heterogeneous nucleation process generated by MgPc particles depends directly on the structure of the doped MgPc and the thermic gradient between the substrate (298 K) and the system doped MgPc-Nylon 11 (573 K). Apparently, due to the lower free energy per unit volume of the sphere, the nuclei of the membrane deposited on the substrate resemble this geometry that, when it grows, is maintained as the deposit advances and the membrane is formed. Inside the membrane are also present particles of amorphous doped semiconductor (
Figure 2a) surrounded by Nylon 11, which generates a heterogeneous morphology. It is worth mentioning that the polymeric matrix is evenly distributed over the substrate (
Figure 2b); this uniformity prevents the dissipation of the electromagnetic radiation that may affect the electrical behavior in the doped MgPc. Thermal relaxation is also an aspect to consider; while heating Nylon 11, fibers undergo an elongation, which allows MgPc doped particles to get into the matrix of the membrane. As the polymer gets cold, it is followed by a contraction of the fibers, and the semiconductor particles remain surrounded by contacted fibers (
Figure 2c). In addition, EDS (Bruker Corporation, Harvard, MA, USA) studies were carried out on the deposited membranes over Corning glass. In
Figure 2c, the presence of different atoms in the semiconductor particles and covered by the Nylon 11 matrix that integrate the membrane is observed. The presence of the magnesium is correspondent to the MPc molecule; nitrogen can be an indication of both the presence of the molecule itself, as well as of Nylon 11, and oxygen is present in both the polymer and the allene. By complementing this information with that reported by IR spectroscopy, it is confirmed that nitrogen is present in the macrocycle by the presence of the bands assigned to the C=N and to the C–N vibration modes. This is also observed in Nylon 11, by the presence of the band referent to the stretching vibrations for N–H. The presence of oxygen in the Nylon 11 is verified by the signal referent to C=O vibration, and in the allene, its presence is confirmed by the band O–H observed in the carboxylic acid.
The MgPc is an organic semiconductor π-conjugated that falls within the category of a discotic system formed by an aromatic nucleus. It has the capacity to organize itself in almost mono-dimensional columns, which allows the charge transport in one direction only. This one-directional charge transport is influenced by the π-stacking interactions between neighbor molecules, and it can be evaluated by the four point probe collinear method. Since the charge carrier density inside the membrane is too low for the transport in the interior, it is necessary to inject charges from an electrode that acts as anode. In this case, a transparent conductor contact of ITO was used to inject holes, while a silver electrode as cathode was used to inject electrons, both as a consequence of the application of an electric field (see
Figure 3a).
J-V characteristic was evaluated in natural light conditions (
Figure 3b), as well as in darkness (
Figure 3c), in order to verify the electric behavior of the membrane before and after the thermal treatment. Both light conditions were used to verify the electromagnetic radiation effect generated on the membrane, which in turn is necessary to establish the type of optoelectronic device that can be used. In
Figure 3b,c, it is observed that the behavior is practically similar: the radiation has no effect in both the semiconductor thin film and the membrane. On the other hand, important differences in the electric behavior of the membrane before and after thermal treatment were observed. Before thermal treatment, the membrane presents a symmetric behavior, similar to the one of the semiconductor thin film. Additionally, at low voltages the behavior is ohmic, while at voltages higher than 1 V a change of slope is shown, referring to an accumulation of charges that are similar to a resistor. After the thermal treatment, with the incorporation of semiconductor particles in the Nylon 11 matrix, a behavior more likely to a light emitting diode is observed [
30]. Apparently, the hole injection is conducted through the ITO, and the electron injection through the Ag. These carriers travel from the electrodes to the membrane, where, apparently, light is produced by the radiative recombination of generated excitons from the injection of the carriers [
30]. It is evident that the thermal relaxation of the membrane generates a change in the electric behavior; however, it is necessary to study this membrane under different electromagnetic radiation conditions.
The electrical characterization of the membrane that was thermally treated was carried out by measuring the
J-V curves in artificial and natural lighting conditions. The latter simulates the irradiation of the membrane with sunlight and in dark conditions with the aim of evaluating the membrane functionality that is analogous to a light emitting diode. The results are shown in
Figure 4a, where it appears that under the presence of different wavelengths of electromagnetic radiation, the membrane shows practically the same behavior. Although, the high energy UV radiation generates the higher density of current transported to the interior of the membrane, while in darkness, the current density drops considerably. Additionally, with respect to the
J-V curves measured under illumination conditions, ohmic behavior is observed, whereas the fact that such low current density in dark conditions was obtained is indicative of high resistance at the interior of the membrane. This resistance can be associated with the presence of Nylon 11, and, as a consequence, the electric behavior of an annealed thin film of doped MPc without Nylon also was analyzed (
Figure 4b) in order to be compared. For voltages lower than 1 V, an ohmic behavior is observed, but when the voltage increases, a change of slope is observed due to an alteration in the regime of charge transport mainly associated with the accumulation of charges inside the semiconductor film. This behavior is present in both measurements performed in dark, as well as under illumination conditions. It is concluded that the presence of Nylon 11 enhances the electric characteristics in the material, although at voltages below 1 V, the behavior is practically ohmic in both the semiconductor without the Nylon and as membrane. By increasing the voltage, a charge accumulation is generated in the semiconductor film and can be controlled by a SCLC type current (space-charge-limited conduction) or also by a model based on cargo traps [
41], while in the other hand, the membrane ohmic behavior is maintained under illumination conditions. Moreover, the presence of the Nylon 11 allowed a broad variation of the current density with the different incident wavelengths that is not observed for the semiconductor film itself.
On the other hand, in
Figure 4a,b the electrical resistivity (
ρ) under the effect of different types of illumination for both membrane and semiconductor film can be observed. It is important to notice that the calculated
ρ is of the same order of magnitude in both cases and that under natural lighting conditions (white labeled in both Figures),
ρ, between the film and the membrane, did not show significant variation. Therefore, apparently Nylon 11 does not generate an electric isolating effect in the membrane. In the range of visible radiation, there are changes due to the effect of polymer on the membrane. In contrast to the film, the
ρ increases when the wavelength is increased due to the shift to low energy (blue radiation to red). On the membrane, the
ρ increases as the wavelength of incident light is decreased from red to blue. However, both in the membrane and in the semiconductor film, the lowest
ρ occurs under the presence of UV illumination. This is related to the density of transported charge carriers, which is superior for the highest energy of UV radiation where a higher generation of charge carriers is presented. The highest
ρ is in dark conditions for both the membrane and the semiconductor film, where, apparently, the rate of generation of charge carriers is lower. It is important to mention that the electric resistivity is the inverse of the conductivity:
Based on the
ρ values obtained for the membrane at room temperature and under ilumination conditions (
Figure 4a), a σ in the range of 19.12 and 49.51 S/cm was obtained. The σ value of the membrane is in the range of the semiconductor materials (10
3 to 10
−8 S/cm) [
30]. Thie results, as expected, are important for the application of the proposed membrane in the manufacture of optoelectronic devices. The latter is because it allows the electric conductivity value of the semiconductor to be maintained as integrated in the membrane. The characterization of the membrane in dark conditions reproduces behavior analogous to that of a simple light emitting diode (OLED). As mentioned above, the injection of charge carriers of opposite electric charges will flow from the electrodes to the membrane. Hereby, the recombination of a generated exciton, electrically from the injection of carriers, may produce light. The electric conductivity obtained for the membrane in dark conditions at room temperature is 15.27 S/cm; this value is also above the organic semiconductors range reported. It is known that the water molecules may reduce the electrical conductivity of the MPcs semiconductor due to its capability to dissolve the material [
42,
43]. The latter is amplified when high energy light is used for charge carrier generation and a diminution of the generated carries is presented. This is as a consequence of an increase of the trapping probability and an electron mobility decrease within the membrane [
44]. As observed in
Figure 4b, there is no such effect in the
J-V characteristics, which in turn indicates that the semiconductor material is isolated from water molecules in the environment.
Additionally, the refractive index (
n) was determined in order to get information about the change in direction/velocity of the electromagnetic radiation experiments, for different types of illumination (natural and artificial), when passing through the membrane. The results obtained by ellipsometry, for both membrane and semiconductor film, are shown in
Table 2. It is observed that the refractive index of the membrane is lower than that of the film and is mainly related to the Nylon 11 fibers that surround the MgPc doped particles. The refractive index value for conventional Nylon 11 is 1.52, and the values obtained are lower than that [
45]. Also, it can be observed that the values of both the film and the membrane are very close to the air refractive index (~1), which indicates that the transmition of the light across the mediums has a small deviation from its original direction and reduces the reflection of the interface between mediums.
In order to analyze the possible photo-degradation of Nylon 11 and its effect on the transport properties in doped MgPc, the membrane was irradiated with a 360 Watts lamp in accelerated conditions during 8 h; then, the presence of main functional groups was evaluated by IR spectroscopy, and finally the
J-V behavior in darkness and under illumination was determined. This behavior was compared to that of the semiconductor film irradiated under the same conditions. In
Figure 5 the IR spectrum of before and after the light irradiation is shown. The curves show signs referent to the stretching vibrations for N–H (around 3308 and 3077 cm
−1) and the band assigned to the C=O vibrations (around 1652 cm
−1) in the Nylon 11. Based on IR spectroscopy, it is possible to conclude that there was not a chemical decomposition of the Nylon in the membrane, nor of the doped semiconductor (the bands corresponding to C–C, C=N and C–N vibrations in the MgPc; the vibrations of the O–H and the C–H bonds of the allene are observed). It is worth mentioning that during the irradiation, the membrane was exposed to environmental conditions such as the presence of air and humidity of the environment. However, the presence of the band assigned to the O–H bond of water in the IR spectrum is not observed. Apparently, Nylon 11 functions as a suitable barrier between the semiconductor and the environmental water. It is worth mentioning the above, since most of the organic semiconductors tend to oxidation and degrade chemically in environmental conditions [
11]. These cause them to lose competitiveness against the inorganic semiconductors like silicon; the fact that the Nylon protects the semiconductor favors the use of these membranes for optoelectronic devices, increasing, as well, its useful life.
Respectively, the
J-V evaluation was done in order to verify the effect of the irradiation on the electric properties of the semiconductor. It is observed in
Figure 6 that, with the exception of the dark condition for membrane, both the film and the membrane itself show a similar behavior for the evaluated voltage range. The latter exists with no significant variation in the current density when the measurement voltage polarity changes. Although, a significant decrease of the current values in the membrane is observed with respect to that obtained before the 8 h of accelerated irradiation. Current density transported in the material is lower for the membrane, except in the case of dark condition, where, practically, the behavior does not change with respect to the one obtained before the irradiation. Besides, there is an increase in the current density that is transported when the voltage increases. Apparently, the membrane maintains an operation similar to that of an OLED [
46,
47,
48,
49]: the MgPc is used as
p type semiconductor, and allene molecules are introduced in the material, in such a way that they contribute to the generation of charge carriers. Then, the presence of both compounds favors the injection and electron-hole transport within the membrane, facilitating the charge balance and their transport between the electrodes. It is worth mentioning that in the case of the semiconductor film the accumulation of charges in the material is reduced with the accelerated irradiation, although the
J values do not exceed those obtained in the membrane. Based on above, it is observed that the presence of Nylon 11 in the membrane does not affect the electrical functionality of the semiconductor in its application to the manufacture of the OLED, and, according to IR spectroscopy, the polymer remarkably protects its functionality against degradation by the presence of humidity in the environment [
11].
The optical absorbance spectra of the membrane and the semiconductor film deposited on quartz were recorded for a wavelength range from 1100 to 200 nm and are shown in
Figure 7. To characterize the optoelectronic properties of the membrane, optical absorption measurements were conducted to determine the parameters that describe the electronic transitions. The differences observed on the absorbance can be attributed to the presence of Nylon 11 in the membrane; the shape of the spectrum is due to MgPc with D
4h symmetry [
50]. The spectral properties of the membrane are caused by the MgPc doped semiconductor, which presents two typical bands: the Q-band and the B-band; these bands confirmed the presence of MgPc doped on the surface of the membrane [
11]. Two peaks for Q-band can be observed in the visible region: a high energy peak, around 640 nm, and a second one, a low energy peak, around 690 nm [
6,
32,
33,
34]. The high-energy peak of the
Q-band is assigned to the first π–π
* transition on the MgPc doped semiconductor. A second π–π
* transition associated with the low-energy peak of the
Q-band is explained as an excitation peak, as a surface state, and as a vibrational internal interval [
33,
34]. The appearance of a red shifted absorption at 640 nm relative to the monomer peak at 690 nm is explained as a result of coplanar association of MgPc macrocycles progressing from monomers and leading to aggregates [
11,
50]. The MgPc rings are arranged in a face-to-face position (H-type) in the aggregate [
50]; however, according to Lapok et al. [
50] in their work related to the use of MgPc on membranes, the spectra do not present information related to the α y β forms of the MgPc. The absorption spectrum of the monoclinic structure (α-form) has a doublet around 708 and 653 nm, while in the tricyclic spectrum of the (β-form) a high intensity band is observed at 646 nm with two shoulders at 620 and 665 nm [
51]. Finally, the B-band is within the UV region of the spectrum, at around 340 nm [
6,
28,
29,
30], and it refers to the electronic
n-π
* transitions between the molecules. The B-band is due to
together with
transitions [
52,
53].
Figure 8a shows the Photoluminescence spectra of the membrane and semiconductor film. The PL spectra were normalized to the thickness of each samples. Four peaks that the film and the membrane have in common can be observed: ~475, ~680, ~720, and ~820 nm. The short wavelength peak in both materials corresponds to the region of no absorption in
Figure 6; however, small band tails are observed. The membrane shows another peak at ~751 nm that is characteristic of the Nylon 11 and disappears for the film. It is important to notice that for the semiconductor film (
Figure 8b), the absorbance values are small compared to those of the membrane. The peak ~680 nm, associated with the π*–π relaxation transitions of the doped MPc macrocycle, is red shifted for the membrane. The last is related to the π–π* stacking of the conjugated MPc, as the semiconductor particles are introduced in the Nylon 11, which infers a molecule aggregation. The membrane narrow peak ~475 nm is mainly related to the Nylon 11 [
54,
55], which in turn is a doublet of the peak ~751 nm optically-allowed exciton recombination. Moreover, doped MPc also contributes to the PL intensity of the previous signal and is dependent on the electron transition to deeper levels [
6,
32]. The broadening of this peak for the semiconductor film may be associated with the Pc orbital overlap with the Mg central atom and with the amorphous nature that allows the formation of delocalized states between HOMO and LUMO. The latter, as a consequence, generates a non-radiative relaxation and it is responsible for the semiconducting properties. On the other hand, the semiconductor film shows a small peak ~590 nm attributed to the Pc [
6] and PL emission at ~430 nm that is identified as singlet exciton recombination. Also, the PL in the visible range was observed with naked eye and varies in colour, red shifted for the membrane, as a consequence of the different peaks intensity. Therefore, a significant change is observed in the PL efficiency and PL maximum position when the MgPc are introduced in the Nylon 11 fibers.
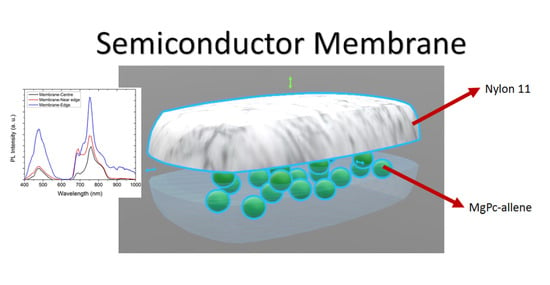
The PL Spectra of the membrane at different positions within the sample are shown on
Figure 8b. First, it can be appreciated that, as the measure is made closer to the edge of the sample, the PL spectra intensity is increased. The peak at approximately 720 nm is more pronounced at the edge. On the other hand, the peak at around 680 nm is sharpened, as the measure is made closer to the edge and it is more intense near the edge (red line curve). All of this indicates that the emission intensity varies along the membrane, but the emission wavelengths remain the same. This could be related to the amount of MgPc doped particles that are incorporated inside the fibers of Nylon 11 during evaporation. Substrate temperature during deposition might have a thermic gradient between the centre and the edge, which explains the increase in the semiconductor particles at the edge. Furthermore, the higher PL intensity could be attributed to the formation of charge transfer complex and to the arrangement of the molecules. It is worth mentioning that positions close to the membrane centre present a PL emission, with minimal variations indicating a homogenous material.
The reflectance (
R) percentage obtained from Equation (2) and the refractive index are shown in
Table 2. The optical properties of both the semiconductor film and the membrane can be analyzed according to the Tauc model, as their estimated reflectance is lower than 15% [
32,
56]. This model is used to determine the optical properties of amorphous semiconductor materials (the presence of Nylon 11 in the matrix of the membrane gives an amorphous array to the structure). The low reflectance percentage allows a large number of photons with different wavelengths to be absorbed.
According to the semi-empirical Tauc model, the optical band gap (
Eg) can be deduced from the UV-Vis absorption spectrum [
32,
56]. In amorphous semiconductors, electronic transitions are described by non-direct transitions with no conservation of electronic momentum. The Tauc optical gap for non-direct transitions could be determined by the extrapolation to zero of the linear regions of the (α
hν)
½ =
f(hν) plots. The absorption coefficient (α) is related to each wavelength that is irradiated in terms of the transmittance (
T) and thickness (τ) of the sample. Film and membrane thickness were obtained by null ellipsometry measurements, as shown in
Table 2.
On the other hand, Equation (4) was used to calculate the photon energy (
Ephoton) for each wavelength (λ), in which
c is the speed of light in the vacuum and
h is Planck’s constant. The optical gap band results are shown in
Table 3, where it can be observed that the membrane presents practically the same optical band gap as the MgPc semiconductor doped with allene.
As expected, the Tauc’s optical gap is lower for the doped MgPc than for the intrinsic, and the optical gap is also higher for the membrane without the thermal treatment than for the membrane after the thermal relaxation. However, the little variation between the optical gap of the membrane and the semiconductor film is a sign of the viability for using the Nylon 11 membrane in optoelectronic applications, even though the final structure array of the membrane is amorphous. The structural disorder due to the weak non-covalent interactions that govern it leads to a not identical environment of each semiconductor molecule. The latter is compared to the other molecules integrated in the membrane, i.e., the energy of the molecules orbitals that form the membrane and cause the molecule to not be isoenergetic, but will present an energy distribution. However, the value obtained from Tauc’s optical gap for the membrane lets us conclude that the semiconductor properties of the doped MgPc were not lost. Comparing the Tauc optical gap shown on
Table 3 to the PL maximum peak energy it can be found that, for the membrane, the indirect bandgap transition is related to the peak of ~475 nm. On the other hand, for the semiconductor film, an indirect bandgap transition is related to the peak ~427 nm.