Precise Dissolution Control and Bioavailability Evaluation for Insoluble Drug Berberine via a Polymeric Particle Prepared Using Supercritical CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Preparation of BPs and ICB
2.3. HPLC Analysis of BBR Content
2.4. Characterization Methods
2.5. Dissolution Study
2.6. Pharmacokinetic Studies in Rats and Data Analysis
3. Results and Discussion
3.1. Particle Morphology Manipulation
3.1.1. Effect of λ
3.1.2. Effect of Temperature, Pressure, and Concentration
3.2. Composition Analysis
3.3. Structure Characterization
3.3.1. IR, XRD, and DSC Characterization
3.3.2. Intermolecular Interaction of BBR and β-CD in BPn
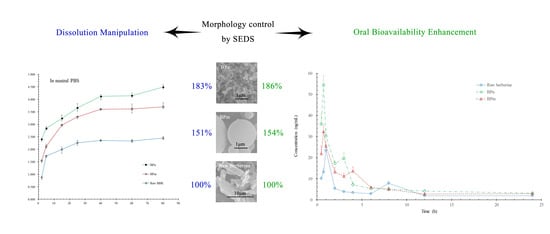
3.4. Dissolution Enhancement and Morphological Dependence
3.5. Pharmacokinetic Studies in Rats
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Explanation of Abbreviations and Symbols
| Abbreviation or Symbol | Full Name |
| AUC | The area under the concentration–time curve |
| AUC0→∞ | AUC from time zero to infinity |
| AUC0→t | AUC from time zero to test time |
| BBR | berberine |
| BP | Berberine-loaded solid polymeric particle |
| BPm | BP of microsphere |
| BPmix | BP of mixed morphology |
| BPn | BP of nanosphere |
| Cmax | Peak concentration |
| DCM | Dichloromethane |
| DMSO | Dimethyl sulfoxide |
| Frel | Relative bioavailability |
| ICB | Traditional inclusion complexes of BBR |
| MRT | Mean residence time |
| PBS | Phosphate-buffered saline |
| SAS | Supercritical antisolvent process |
| SEDS | Solution-enhanced dispersion by supercritical fluids |
| Tmax | Time of peak concentration |
| β-CD | β-cyclodextrin |
| λ | Initial mol ratio of BBR to β-CD |
| λ′ | Mol ratio of BBR to β-CD in product |
References
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Dai, Y.; Verpoorte, R.; Lam, W.; Cheng, Y.C.; Pao, L.H.; Zhang, W.; Chen, S. Major Achievements of Evidence-Based Traditional Chinese Medicine in Treating Major Diseases. Biochem. Pharmacol. 2017, 139, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.R.; De Andrade Neto, J.B.; Da Silva, C.R.; Campos, R.D.S.; Costa Silva, R.A.; Freitas, D.D.; Do Nascimento, F.B.S.A.; De Andrade, L.N.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Yang, S.; Wang, Y.; Huang, Z.; Gao, J.; Tu, S.; Rao, Z. Berberine Ameliorates Collagen-Induced Arthritis in Rats Associated with Anti-Inflammatory and Anti-Angiogenic Effects. Inflammation 2014, 37, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The Anti-Inflammatory Potential of Berberine in Vitro and in Vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Pires, C.; Hostettmann, K.; Haouala, A.; Cuendet, M.; Falquet, J.; Graz, B.; Christen, P. Reverse Pharmacology for Developing an Anti-Malarial Phytomedicine. The Example of Argemone Mexicana. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yang, X.; Liu, Y.; Zhang, Y.; Wang, P.; Wang, J. Chinese Herbal Formulas for Treating Hypertension in Traditional Chinese Medicine: Perspective of Modern Science. Hypertens. Res. 2013, 36, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.J.; Ding, H.H.; Tang, L.Q. Berberine as a Promising Anti-Diabetic Nephropathy Drug: An Analysis of its Effects and Mechanisms. Eur. J. Pharmacol. 2015, 760, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Vaid, M.; Katiyar, N.; Sharma, S.; Katiyar, S.K. Berberine, an Isoquinoline Alkaloid, Inhibits Melanoma Cancer Cell Migration by Reducing the Expressions of Cyclooxygenase-2, Prostaglandin E2 and Prostaglandin E2 Receptors. Carcinogenesis 2011, 32, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Yang, J.S.; Li, T.C.; Lin, J.J.; Lin, J.G.; Lai, K.C.; Ma, C.Y.; Wood, W.G.; Chung, J.G. Berberine Suppresses in Vitro Migration and Invasion of Human SCC-4 Tongue Squamous Cancer Cells through the Inhibitions of FAK, IKK, NF-κB, u-PA and MMP-2 and -9. Cancer Lett. 2009, 279, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a Novel Cholesterol-Lowering Drug Working through a Unique Mechanism Distinct from Statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a Plant Alkaloid with Lipid- and Glucose-Lowering Properties: From in Vitro Evidence to Clinical Studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research Progress on Berberine with a Special Focus On its Oral Bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, G.; Wu, T.; Bai, F.; Xu, J.; Zhang, X. Solid Dispersion of Berberine Hydrochlorid and Eudragit® S100: Formulation, Physicochemical Characterization and Cytotoxicity Evaluation. J. Drug Deliv. Sci. Technol. 2017, 40, 21–27. [Google Scholar] [CrossRef]
- Zhaojie, M.; Ming, Z.; Shengnan, W.; Xiaojia, B.; Hatch, G.M.; Jingkai, G.; Li, C. Amorphous Solid Dispersion of Berberine with Absorption Enhancer Demonstrates a Remarkable Hypoglycemic Effect Via Improving its Bioavailability. Int. J. Pharmaceut. 2014, 467, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wu, X.; Dong, W.; Sun, W.; Li, J.; Tang, X. Enhancement by Sodium Caprate and Sodium Deoxycholate of the Gastrointestinal Absorption of Berberine Chloride in Rats. Drug Dev. Ind. Pharm. 2013, 39, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Yang, Y.; Dai, Y.; Li, Q.; Lin, G.; Ma, Y.M. Polyethylene Glycol 400 (PEG400) Affects the Systemic Exposure of Oral Drugs Based on Multiple Mechanisms: Taking Berberine as an Example. RSC Adv. 2017, 7, 2435–2442. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS On Intestinal Absorption in Rats. AAPS Pharmscitech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.L.; Gao, L.N.; Jiang, H.L. Effects of B-Cyclodextrin On the Intestinal Absorption of Berberine Hydrochloride, a P-glycoprotein Substrate. Int. J. Biol. Macromol. 2013, 59, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Qin, Q.; Liao, X.; Yang, B. Host-Guest Inclusion System of Glycyrrhetic Acid with Polyamine-B-Cyclodextrin: Preparation, Characterization, and Anticancer Activity. J. Mol. Struct. 2017, 1149, 155–161. [Google Scholar] [CrossRef]
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A Novel Multi-Tiered Experimental Approach Unfolding the Mechanisms Behind Cyclodextrin-Vitamin Inclusion Complexes for Enhanced Vitamin Solubility and Stability. Int. J. Pharmaceut. 2017, 532, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Tang, D.; Feng, L.; Zheng, Z.G.; Wang, R.S.; Wu, A.G.; Duan, T.T.; He, B.; Zhu, Q. Development of Self-Microemulsifying Drug Delivery System for Oral Bioavailability Enhancement of Berberine Hydrochloride. Drug Dev. Ind. Pharm. 2013, 39, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, N.; Song, H.; Xi, X.; Wang, J.; Hao, A.; Li, T. Preparation of an Anhydrous Reverse Micelle Delivery System to Enhance Oral Bioavailability and Anti-Diabetic Efficacy of Berberine. Eur. J. Pharm. Sci. 2011, 44, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, M.X.; Zhang, W.; Li, X.M.; Gao, D.H.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Characterization, Pharmacokinetics, and Hypoglycemic Effect of Berberine Loaded Solid Lipid Nanoparticles. Int. J. Nanomed. 2013, 2013, 4677–4687. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.P.; Wang, X.; Wang, H.; Chen, T.; Wang, Y.; Wang, Z. In Vitro Antitumor Efficacy of Berberine: Solid Lipid Nanoparticles Against Human HepG2, Huh7 and EC9706 Cancer Cell Lines. In Colloidal Nanoparticles for Biomedical Applications XI; 97221A; SPIE: Bellingham, WA, USA, 2016. [Google Scholar]
- Esfandiari, N. Production of Micro and Nano Particles of Pharmaceutical by Supercritical Carbon Dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical Fluids Applications in Nanomedicine. J. Supercrit. Fluids 2015, 101, 193–214. [Google Scholar] [CrossRef]
- Jia, J.; Wang, J.; Zhang, K.; Zhou, D.; Ge, F.; Zhao, Y. Aescin Nanoparticles Prepared Using SEDS: Composition Stability and Dissolution Enhancement. J. Supercrit. Fluids 2017, 130, 267–272. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Prosapio, V.; Cice, F.; Reverchon, E. Use of Solvent Mixtures in Supercritical Antisolvent Process to Modify Precipitates Morphology: Cellulose Acetate Microparticles. J. Supercrit. Fluids 2013, 83, 153–160. [Google Scholar] [CrossRef]
- Rossmann, M.; Braeuer, A.; Leipertz, A.; Schluecker, E. Manipulating the Size, the Morphology and the Polymorphism of Acetaminophen Using Supercritical Antisolvent (SAS) Precipitation. J. Supercrit. Fluids 2013, 82, 230–237. [Google Scholar] [CrossRef]
- Jia, J.; Song, N.; Gai, Y.; Zhang, L.; Zhao, Y. Release-Controlled Curcumin Proliposome Produced by Ultrasound-Assisted Supercritical Antisolvent Method. J. Supercrit. Fluids 2016, 113, 150–157. [Google Scholar] [CrossRef]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of Lutein and Co-Precipitation of Lutein and Poly-Lactic Acid with the Supercritical Anti-Solvent Process. Chem. Eng. Process. Process Intensif. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- De Marco, I.; Rossmann, M.; Prosapio, V.; Reverchon, E.; Braeuer, A. Control of Particle Size, at Micrometric and Nanometric Range, Using Supercritical Antisolvent Precipitation from Solvent Mixtures: Application to PVP. Chem. Eng. J. 2015, 273, 344–352. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Influence of Pressure, Temperature and Concentration on the Mechanisms of Particle Precipitation in Supercritical Antisolvent Micronization. J. Supercrit. Fluids 2011, 58, 295–302. [Google Scholar] [CrossRef]
- De Marco, I.; Knauer, O.; Cice, F.; Braeuer, A.; Reverchon, E. Interactions of Phase Equilibria, Jet Fluid Dynamics and Mass Transfer During Supercritical Antisolvent Micronization: The Influence of Solvents. Chem. Eng. J. 2012, 203, 71–80. [Google Scholar] [CrossRef]
- Li, N.; Xu, L. Thermal Analysis of β-cyclodextrin/Berberine Chloride Inclusion Compounds. Thermochim. Acta 2010, 499, 166–170. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M. Monodisperse Microparticles Loaded with the Self-Assembled Berberine-Phospholipid Complex-Based Phytosomes for Improving Oral Bioavailability and Enhancing Hypoglycemic Efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Soares, J.M.D.; Silva, M.G.E.; de Lavor, É.M.; Andrade, V.M.; Menezes, P.D.P.; Araújo, A.A.D.S.; Leite, L.H.I.; Menezes, I.R.A.D. Docking, Characterization and Investigation of B-Cyclodextrin Complexed with Farnesol, an Acyclic Sesquiterpene Alcohol, Produces Orofacial Antinociceptive Profile in Experimental Protocols. Process Biochem. 2017, 62, 193–204. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. AAPS Pharmscitech 2010, 11, 1466–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, S.; Hossain, M.; Kumar, G.S. Studies On A-, B-, and Γ-Cyclodextrin Inclusion Complexes of Isoquinoline Alkaloids Berberine, Palmatine and Coralyne. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 311–323. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Shang, X.; Yan, F. Formulation of Berberine Hydrochloride and Hydroxypropyl-B-Cyclodextrin Inclusion Complex with Enhanced Dissolution and Reduced Bitterness. Trop. J. Pharm. Res. 2012, 11, 871–877. [Google Scholar] [CrossRef]
- Bunjes, H. Lipid Nanoparticles for the Delivery of Poorly Water-Soluble Drugs. J. Pharm. Pharmacol. 2010, 62, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Improved Delivery of Poorly Soluble Compounds Using Nanoparticle Technology: A Review. Drug Deliv. Transl. Res. 2016, 6, 319–332. [Google Scholar] [CrossRef] [PubMed]









| Sample No. | λ (n/n) | Temperature (°C) | Pressure (bar) | BBR Concentration (mg/mL) | λ′ (n/n) | SEM Images |
|---|---|---|---|---|---|---|
| 1 | 1:2 | 40 | 120 | 15 | 0.46 | Figure 2a |
| 2 | 1:1 | 40 | 120 | 15 | 1.02 | Figure 2b |
| 3 | 3:1 | 40 | 120 | 15 | 2.10 | Figure 2c |
| 4 | 5:1 | 40 | 120 | 15 | 3.52 | Figure 2d |
| 5 | BBR only | 40 | 120 | 15 | \ | Figure 2e |
| 6 | 1:1 | 50 | 120 | 15 | 1.03 | Figure 3a |
| 7 | 1:1 | 60 | 120 | 15 | 0.93 | Figure 3b |
| 8 | 1:1 | 40 | 90 | 15 | 0.94 | Figure 3c |
| 9 | 1:1 | 40 | 150 | 15 | 1.03 | Figure 3d |
| 10 | 1:1 | 40 | 120 | 9 | 0.95 | Figure 3e |
| 11 | 1:1 | 40 | 120 | 3 | 0.88 | Figure 3f |
| δ | Δδ | |||||
|---|---|---|---|---|---|---|
| δ(β-CD) | δ(BPn) | δ(ICB) | δ(BPn) − δ(β-CD) | δ(ICB) − δ(β-CD) | ||
| H-1 | d | 3.99 | 4.00 | 3.99 | 0.01 | 0.00 |
| H-2 | t | 3.51 | 3.50 | 3.50 | −0.01 | −0.01 |
| H-3 | t | 3.89 | 3.83 | 3.82 | −0.06 | −0.07 |
| H-4 | dd | 3.57 | 3.58 | 3.59 | 0.01 | 0.02 |
| H-5 | m | 3.78 | 3.74 | 3.72 | −0.04 | −0.06 |
| H-6 | m | 3.82 | 3.79 | 3.78 | −0.03 | −0.04 |
| Parameters | Raw Berberine | BPm | BPn |
|---|---|---|---|
| Cmax (μg/mL) | 25.5 ± 5.5 | 33.1 ± 5.9 | 54.4 ± 10.3 |
| Tmax (h) | 1.0 ± 0.00 | 0.85 ± 0.14 | 0.75 ± 0.01 |
| AUC0→t (μg·L−1·h) | 92.5 ± 9.6 | 142.1 ± 10.7 | 171.7 ± 12.5 |
| AUC0→∞ (μg·L−1·h) | 149.7 ± 11.9 | 173.6 ± 5.0 | 207.1 ± 21.3 |
| K (h−1) | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.01 |
| t1/2 (h) | 12.70 ± 2.00 | 7.54 ± 1.42 | 7.88 ± 1.50 |
| CL (L·kg−1·h−1) | 67.15 ± 5.42 | 57.63 ± 1.63 | 48.68 ± 4.87 |
| MRT (h) | 18.33 ± 2.88 | 10.89 ± 2.05 | 11.38 ± 2.16 |
| Frel (%) | 100 | 153.59 ± 11.53 | 185.57 ± 13.49 |
| (n = 5, mean ± SD) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Zhang, K.; Zhou, X.; Zhou, D.; Ge, F. Precise Dissolution Control and Bioavailability Evaluation for Insoluble Drug Berberine via a Polymeric Particle Prepared Using Supercritical CO2. Polymers 2018, 10, 1198. https://doi.org/10.3390/polym10111198
Jia J, Zhang K, Zhou X, Zhou D, Ge F. Precise Dissolution Control and Bioavailability Evaluation for Insoluble Drug Berberine via a Polymeric Particle Prepared Using Supercritical CO2. Polymers. 2018; 10(11):1198. https://doi.org/10.3390/polym10111198
Chicago/Turabian StyleJia, Jingfu, Kerong Zhang, Xue Zhou, Dan Zhou, and Fahuan Ge. 2018. "Precise Dissolution Control and Bioavailability Evaluation for Insoluble Drug Berberine via a Polymeric Particle Prepared Using Supercritical CO2" Polymers 10, no. 11: 1198. https://doi.org/10.3390/polym10111198
APA StyleJia, J., Zhang, K., Zhou, X., Zhou, D., & Ge, F. (2018). Precise Dissolution Control and Bioavailability Evaluation for Insoluble Drug Berberine via a Polymeric Particle Prepared Using Supercritical CO2. Polymers, 10(11), 1198. https://doi.org/10.3390/polym10111198