Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2
Abstract
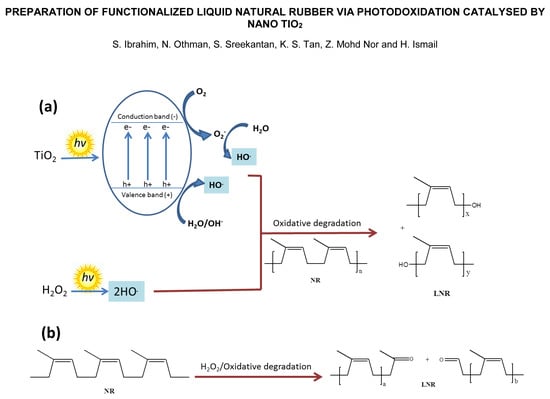
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 via Sol–Gel Method
2.3. Photodegradation of NR Latex
2.4. Characterizations
3. Results and Discussion
3.1. Preparation and Characterization of TiO2 Nanocrystals
3.1.1. Effect of pH
3.1.2. Effect of Annealing Temperature
3.2. Photodegradation of NR Latex
3.2.1. Effect of TiO2 Nanocrystals on Photodegradation
3.2.2. FTIR Analysis of LNRs
3.2.3. NMR Analysis of LNRs
3.2.4. SEM and TEM Micrographs of Latex Particles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Wang, R.; Zhang, L. Editorial corner—A personal view status, challenges and future trends of biorubber. Express Polym. Lett. 2017, 11, 343. [Google Scholar] [CrossRef]
- Cornish, K. Biochemistry of natural rubber, a vital raw material, emphasizing biosynthetic rate, molecular weight and compartmentalization, in evolutionarily divergent plant species. Nat. Prod. Rep. 2001, 18, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Heping, Y.; Sidong, L.; Zheng, P. Preparation and study of epoxidized natural rubber. J. Therm. Anal. Calorim. 1999, 58, 293–299. [Google Scholar] [CrossRef]
- Le Xuan, H.; Decker, C. Photocrosslinking of acrylated natural rubber. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 769–780. [Google Scholar] [CrossRef]
- Thitithammawong, A.; Srangkhum, S.; Rungvichaniwat, A. Hydroxytelechelic Natural Rubber from Natural Rubber and Epoxidised Natural Rubber. J. Rubber Res. 2011, 14, 230–240. [Google Scholar]
- Amnuaysin, T.; Buahom, P.; Areerat, S. Preparation of natural rubber-based polyol by oxidative degradation under supercritical carbon dioxide for flexible bio-based polyurethane foams. J. Cell. Plast. 2015, 52, 585–594. [Google Scholar] [CrossRef]
- Hirzin, R.S.F.N.; Azzahari, A.D.; Yahya, R.; Hassan, A.; Tahir, H. The behavior of semi-rigid polyurethane film based on functionalized rubber by one-shot and two-shot method preparation. J. Mater. Sci. 2018, 53, 13280–13290. [Google Scholar] [CrossRef]
- Baharulrazi, N.; Nor, H.M.; Ali, W.K.W. Hydroxyl Terminated Natural Rubber (HTNR) as a Binder in Solid RocketPropellant. Appl. Mech. Mater. 2015, 695, 174–178. [Google Scholar] [CrossRef]
- Klinpituksa, P.; Rungvichaniwat, A.; Saetung, A.; Pilard, J.F.; Campistron, I.; Laguerre, A. Polyurethane Adhesives from Hydroxyl Terminated Natural Rubber. J. Rubber Res. 2012, 15, 217–229. [Google Scholar]
- Kwanming, K.; Klinpituksa, P.; Waehamad, W.-A. Ultraviolet Curing of Acrylated Liquid Natural Rubber for Surface Coating Application. Songklanakarin J. Sci. Technol. 2008, 311, 49–55. [Google Scholar]
- Karnika De Silva, K.G.; Silva, E.; Vitharana, L.P. Depolymerized Natural Rubber as a Processing Aid. J. Rubber Res. Inst. Sri Lanka 1996, 77, 38–53. [Google Scholar]
- Nair, N.R.; Thomas, S.; Mathew, N.M. Liquid Natural Rubber as a Viscosity Modifier in Nitrile Rubber Processing. Polym. Int. 1997, 42, 289–300. [Google Scholar] [CrossRef]
- Dahlan, H.M.; Zaman, M.D.K.; Ibrahim, A. Liquid Natural Rubber (LNR) as a Compatibilizer in NR/LLDPE Blends. J. Appl. Polym. Sci. 2000, 78, 1776–1782. [Google Scholar] [CrossRef]
- Chaiyasat, A.; Waree, C.; Songkhamrod, K.; Sirithip, P.; Voranuch, V.; Chaiyasat, P. Preparation of polydivinylbenzene/natural rubber capsule encapsulating octadecane: Influence of natural rubber molecular weight and content. Express Polym. Lett. 2012, 6, 70–77. [Google Scholar] [CrossRef]
- Panwiriyarat, W.; Tanrattanakul, V.; Pilard, J.F.; Pasetto, P.; Khaokong, C. Effect of the Diisocyanate Structure and the Molecular Weight of Diols on Bio-Based Polyurethanes. J. Appl. Polym. Sci. 2013, 130, 453–462. [Google Scholar] [CrossRef]
- Saetung, A.; Rungvichaniwat, A.; Campistron, I.; Klinpituksa, P.; Laguerre, A.; Phinyocheep, P.; Pilard, J.F. Controlled Degradation of Natural Rubber and Modification of the Obtained Telechelic Oligoisoprenes: Preliminary Study of their Potentiality as Polyurethane Foam Precursors. J. Appl. Polym. Sci. 2010, 117, 1279–1289. [Google Scholar] [CrossRef]
- Kumar, K.D.; Kothandaraman, B. Modification of (DGEBA) epoxy resin with maleated depolymerised natural rubber. Express Polym. Lett. 2008, 2, 302–311. [Google Scholar] [CrossRef]
- Panwiriyarat, W.; Saetung, N.; Badawy, H.; Khaokong, C.; Pasetto, P.; Campistron, I.; Nourry, A.; Pascual, S.; Fontaine, L.; Cutrigth, T.; et al. Natural Rubber: An Old Material for New Applications. In Proceedings of the 182nd Technical Meeting of the ACS Rubber Division, Cincinnati, OH, USA, 9 October 2012. [Google Scholar]
- Sakdapipanich, J.; TSuksawad, P.; Insom, K.; Kawahara, S. Preparation of Functionalized Low Molecular Weight Natural Rubber Latex using Solid Nanometric TiO2 Film as a Photocatalyst. Rubber Chem. Technol. 2005, 72, 597–605. [Google Scholar] [CrossRef]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. A novel Method for the Preparation of Hydroxyl Terminated Liquid Natural Rubber. Macromol. Chem. Rapid Commun 1986, 7, 159–163. [Google Scholar] [CrossRef]
- Panwiriyarat, W.; Tanrattanakul, V.; Pilard, J.F.; Pasetto, P.; Khaokong, C. Preparation and Properties of Bio-based Polyurethane Containing Polycaprolactone and Natural Rubber. J. Polym. Environ. 2013, 21, 807–815. [Google Scholar] [CrossRef]
- Gopakumar, S.; Gopinathan Nair, M.R. Determination of Molecular Parameters of NR/PU Block Copolymers by Transport Studies. Eur. Polym. J. 2005, 41, 2002–2009. [Google Scholar] [CrossRef]
- Wayakron Phetphaisit, C.; Bumee, R.; Namahoot, J.; Ruamcharoen, J.; Ruamcharoen, P. Polyurethane Polyester Elastomer: Innovative Environmental Friendly Wood Adhesive from Modified PETs and Hydroxyl Liquid Natural Rubber Polyols. Int. J. Adhes. Adhes. 2013, 41, 127–131. [Google Scholar] [CrossRef]
- Sakdapipanich, J.; Kowitteerawut, T.; Kawahara, S.; Tanaka, Y. Depolymerisation of Highly Purified Natural Rubber. I. Metal-catalysed Oxidation of Deproteinised Natural Rubber. J. Rubb. Res 2001, 4, 1–10. [Google Scholar]
- Ibrahim, S.; Othman, N.; Ismail, H. Degradation of Natural Rubber Latex. In Natural Rubber: Properties, Behavior and Application; Hamilton, J.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 105–136. [Google Scholar]
- Ibrahim, S.; Othman, N.; Mohd Nor, Z.; Ismail, H. Preliminary Study on Photochemical Degradation of Natural Rubber Latex. Macromol. Symp. 2017, 371, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Meera, A.P.; Said, S.; Grohens, Y.; Luyt, A.S.; Thomas, S. Tensile Stress Relaxation Studies of TiO2 and Nanosilica Filled Natural Rubber Composites. Ind. Eng. Chem. Res. 2009, 48, 3410–3416. [Google Scholar] [CrossRef]
- Meera, A.P.; Tlili, R.; Boudenne, A.; Ibos, L.; Poornima, V.; Thomas, S.; Candau, Y. Thermophysical and Mechanical Properties of TiO2 and silica Nanoparticle-Filled Natural Rubber Composites. J. Elastomers Plast. 2012, 44, 369–382. [Google Scholar] [CrossRef]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-Protection and Antibacterial Properties of Natural Rubber/Rutile-TiO2 Nanocomposites. Polym. Degrad. Stab. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- Lin, G.; Tian, M.; Lu, Y.L.; Zhang, X.; Zhang, L.Q. Morphology, Antimicrobial and Mechanical Properties of Nano-TiO2/Rubber Composites Prepared by Direct Blending. Polym. J. 2006, 38, 498–502. [Google Scholar] [CrossRef]
- Mehrizad, A.; Gharbani, P.; Tabatabii, S.M. Synthesis of Nanosized TiO2 Powder by Sol-Gel Method in Acidic Conditions. J. Iran. Chem. Res. 2009, 2, 145–149. [Google Scholar]
- Lim, C.S.; Ryu, J.H.; Kim, D.-H.; Cho, S.-Y.; Oh, W.-C. Reaction Morphology and the Effect of pH on the Preparation of TiO2 Nanoparticles by a Sol-Gel Method. J. Ceram. Process. Res. 2010, 11, 736–741. [Google Scholar]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol-Gel Synthesised TiO2 Photocatalysts Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol-Gel-Synthesized. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Cenovar, A.; Paunovic, P.; Grozdanov, A.; Makreski, P.; Fidancevska, E. Preparation of Nano-Crystalline TiO2 by Sol-Gel Method using Titanium Tetraisopropoxide (TTIP) as a Precursor. Adv. Nat. Sci. Theory Appl. 2012, 1, 133–142. [Google Scholar]
- Wetchakun, N.; Phanichphant, S. Effect of Temperature on the Degree of Anatase-Rutile Transformation in Titanium Dioxide Nanoparticles Synthesized by the Modified Sol-Gel Method. Curr. Appl. Phys. 2008, 8, 343–346. [Google Scholar] [CrossRef]
- Sayilkan, F.; Asilturk, M.; Sayilkan, H.; Onal, Y.; Akarsu, M.; Arpaç, E. Characterization of TiO2 Synthesized in Alcohol by a Sol-Gel Process: The Effects of Annealing Temperature and Acid Catalyst. Turk. J. Chem. 2005, 29, 697–706. [Google Scholar]
- Simonsen, M.E.; Søgaard, E.G. Sol-Gel Reactions of Titanium Alkoxides and Water: Influence of pH and Alkoxy Group on Cluster Formation and Properties of the Resulting Products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef]
- Marchisio, D.L.; Omegna, F.; Barresi, A.A.; Bowen, P. Effect of Mixing and Other Operating Parameters in Sol-Gel Processes. Ind. Eng. Chem. Res 2008, 47, 7202–7210. [Google Scholar] [CrossRef]
- Kallala, M.; Jullien, R.; Cabane, B. Crossover from Gelation to Precipitation. J. Phys. II 1992, 2, 7–25. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is Anatase a Better Photocatalyst than Rutile? Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, K.; Shi, R.; Li, X.; Dong, X.; Yu, Y. Sol-Gel Synthesis of TiO2 Nanoparticles and Photocatalytic Degradation of Methyl Orange in Aqueous TiO2 Suspensions. J. Alloys Compd. 2006, 413, 302–306. [Google Scholar] [CrossRef]
- Karami, A. Synthesis of TiO2 Nano Powder by the Sol-Gel Method and its use as a Photocatalyst. J. Iran. Chem. Soc. 2010, 7, 154–160. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic Properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. Production of Hydroxyl-Terminated Liquid Natural Rubber—Mechanism of Photochemical Depolymerization and Hydroxylation. J. Appl. Polym. Sci. 1988, 35, 1227–1239. [Google Scholar] [CrossRef]
- Tang, D.K.; Ho, S.Y. The Photopolymerization of Isoprene with the Use of Hydrogen Peroxide as Photoinitiator. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 1357–1363. [Google Scholar] [CrossRef]
- Bussière, P.O.; Gardette, J.L.; Lacoste, J.; Baba, M. Characterization of Photodegradation of Polybutadiene and Polyisoprene: Chronology of Crosslinking and Chain-Scission. Polym. Degrad. Stab. 2005, 88, 182–188. [Google Scholar] [CrossRef]
- Kehlet, C.; Catalano, A.; Dittmer, J. Degradation of Natural Rubber in Works of Art Studied by Unilateral NMR and High Field NMR Spectroscopy. Polym. Degrad. Stab. 2014, 107, 270–276. [Google Scholar] [CrossRef]
- Tasakorn, P.; Amatyakul, W. Photochemical Reduction of Molecular Weight and Number of Double Bonds in Natural Rubber Film. Korean J. Chem. Eng. 2008, 25, 1532–1538. [Google Scholar] [CrossRef]
- Morand, J.L. Chain Scission in the Oxidation of Polyisoprene. Rubber Chem. Technol. 1976, 50, 373–396. [Google Scholar] [CrossRef]
- Ranby, B. Basic Reactions in the Photodegradation of Some Important Polymers. J. Macromol. Sci. Pure Appl. Chem. 1993, A30, 583–594. [Google Scholar] [CrossRef]
- Nagai, N.; Matsunobe, T.; Imai, T. Infrared Analysis of Depth Profiles in UV-Photochemical Degradation of Polymers. Polym. Degrad. Stab. 2005, 88, 224–233. [Google Scholar] [CrossRef]
- Saquib, M.; Abu Tariq, M.; Haque, M.M.; Muneer, M. Photocatalytic Degradation of Disperse Blue 1 using UV/TiO2/H2O2 Process. J. Environ. Manag. 2008, 88, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.H.; Mutsaers, C.A.H.A. Adsorption of Hydrogen Peroxide on the Surface of Titanium Dioxide. J. Phys. Chem. 1975, 79, 1940–1943. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic Production of Hydrogen Peroxides and Organic Peroxides in Aqueous Suspensions of Titanium Dioxide, Zinc Oxide and Desert Sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef] [Green Version]
- Chaikumpollert, O.; Sae-Heng, K.; Wakisaka, O.; Mase, A.; Yamamoto, Y.; Kawahara, S. Low Temperature Degradation and Characterization of Natural Rubber. Polym. Degrad. Stab. 2011, 96, 1989–1995. [Google Scholar] [CrossRef]
- Mohd Nor, H.; Ebdon, J.R. Telechelic Liquid Natural Rubber: A Review. Prog. Polym. Sci. 1998, 23, 143–177. [Google Scholar] [CrossRef]
- Tobolsky, A.V.; Mercurio, A. Catalyzed Oxidative Degradation of Natural Rubber Networks. J. Am. Chem. Soc. 1959, 81, 5539–5540. [Google Scholar] [CrossRef]
- Phetphaisit, C.W.; Phinyocheep, P. Kinetics and Parameters Affecting Degradation of Purified Natural Rubber. J. Appl. Polym. Sci. 2003, 90, 3546–3555. [Google Scholar] [CrossRef]
- Rasid, H.M.; Azhar, N.H.A.; Yusoff, S.F.M. Physicochemical Properties of Liquid Natural Rubber Bearing Fluoro Groups for Hydrophobic Surfaces. J. Polym. Res. 2017, 24, 106. [Google Scholar] [CrossRef]
- Hirzin, R.S.F.N.; Azzahari, A.D.; Yahya, R.; Hassan, A. Optimizing the Usability of Unwanted Latex Yield by in Situ Depolymerization and Functionalization. Ind. Crops Prod. 2015, 74, 773–783. [Google Scholar] [CrossRef]
- Tangpakdee, J.; Mizokoshi, M.; Endo, A.; Tanaka, Y. Novel Method for Preparation of Low Molecular Weight Natural Rubber Latex. Rubber Chem. Technol. 1998, 71, 795–802. [Google Scholar] [CrossRef]
- Rooshenass, P.; Yahya, R.; Gan, S.N. Comparison of Three Different Degradation Methods To Produce Liquid Epoxidized Natural Rubber. Rubber Chem. Technol. 2016, 89, 177–198. [Google Scholar] [CrossRef]
- Rooshenass, P. Investigation of Different Degradation Methods to Prepare Liquid Epoxidized Natural Rubber for Coating Applications; University of Malaya: Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Thuong, N.T.; Yamamoto, Y.; Nghia, P.T.; Kawahara, S. Analysis of damage in commercial natural rubber through NMR spectroscopy. Polym. Degrad. Stab. 2016, 123, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Sansatsadeekul, J.; Sakdapipanich, J.; Rojruthai, P. Characterization of Associated Proteins and Phospholipids in Natural Rubber Latex. J. Biosci. Bioeng. 2011, 111, 628–634. [Google Scholar] [CrossRef] [PubMed]









| TiO2 (phr) | Mn (×103) (g/mol) | Standard Deviation of Mn | Polydispersity | Gel Content (%) | Standard Deviation of Gel Content |
|---|---|---|---|---|---|
| NR | 549.3 | 3.20 | 7.5 | 19.2 | 1.69 |
| 0 | 18.6 | 1.09 | 5.00 | 0.09 | 3.53 |
| 0.2 | 19.1 | 0.88 | 3.01 | 0.24 | 0.11 |
| 0.4 | 15.8 | 1.35 | 3.60 | 0.49 | 0.60 |
| 0.8 | 7.3 | 2.34 | 8.00 | 0.57 | 0.48 |
| 1.2 | 12.7 | 0.16 | 6.08 | 0.69 | 0.27 |
| 1.6 | 20.1 | 0.86 | 5.19 | 0.93 | 0.18 |
| Peaks (cm−1) | Peak Assignment | Peak Area | ||||
|---|---|---|---|---|---|---|
| NR | Amount of TiO2 (phr) | |||||
| 0 | 0.4 | 0.8 | 1.2 | |||
| 3400 | C-OH stretching | 0.211 | 0.960 | 0.694 | 1.699 | 0.865 |
| 1720 | Carbonyl | 0.002 | 0.085 | 0.056 | 0.114 | 0.119 |
| 1661 | C=C stretching | 0.809 | 0.388 | 0.325 | 0.490 | 0.455 |
| 1445 | CH2 bending | 3.712 | 3.446 | 3.493 | 3.397 | 3.455 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.; Othman, N.; Sreekantan, S.; Tan, K.S.; Mohd Nor, Z.; Ismail, H. Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers 2018, 10, 1216. https://doi.org/10.3390/polym10111216
Ibrahim S, Othman N, Sreekantan S, Tan KS, Mohd Nor Z, Ismail H. Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers. 2018; 10(11):1216. https://doi.org/10.3390/polym10111216
Chicago/Turabian StyleIbrahim, Suhawati, Nadras Othman, Srimala Sreekantan, Kim Song Tan, Zairossani Mohd Nor, and Hanafi Ismail. 2018. "Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2" Polymers 10, no. 11: 1216. https://doi.org/10.3390/polym10111216
APA StyleIbrahim, S., Othman, N., Sreekantan, S., Tan, K. S., Mohd Nor, Z., & Ismail, H. (2018). Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers, 10(11), 1216. https://doi.org/10.3390/polym10111216