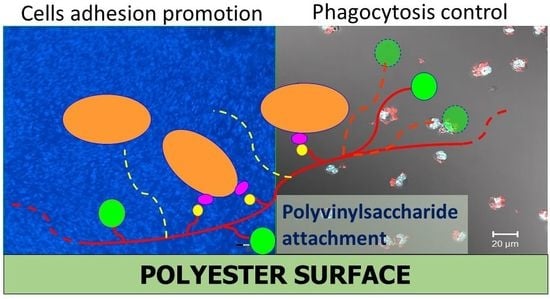
Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Chemical Methods
2.3.1. Materials Preparation
Supermacroporous Matrices
Nanoparticles
2.3.2. Supermacroporous Matrices Modification
Matrix Carboxylation
Matrix Amination
Kinetics of Chemisorption of Ox-PMAG on the Surface of Aminated Matrices (PLA-NH2 and PCL-NH2)
Isotherm of Chemisorption of Ox-PMAG on the Surface of PLA-NH2 and PCL-NH2 Materials
Ox-PMAG-Protein Conjugates Synthesis
Kinetics of CTRG-Cy3 Chemisorption on Polyester Matrix Surface Modified by Aldehyde Containing Ox-PMAG
Kinetics of Ox-PMAG-CTRG Conjugate Chemisorption on Amino-Polyester Matrix
Isotherm of CTRG-Cy3 Chemisorption on the Polyester Matrix Surface Modified by Aldehyde Containing Ox-PMAG
Determination of Unreacted Aldehyde Groups’ Quantity Remained on the Surface of PLA/PCL-NH-PMAG-CTRG Matrices
2.3.3. Nanoparticles Modification
2.4. Cell Culture Experiments
2.4.1. Cell Culture Experiments for Scaffolds Testing
Adhesion Experiment
Static Conditions
Dynamic Conditions
2.4.2. Ex Vivo Determination of Particles Phagocytosis by Peritoneal Macrophages
2.5. Statistical Analysis
3. Results and Discussion
3.1. Materials Formation
3.2. Materials Modification
3.3. Biofunctionalization of Polyester Surfaces with Application of Ox-PMAG
3.3.1. Supermacroporous Matrices
3.3.2. Nanoparticles
3.4. Study of the Effect of Bioligands Attachment on Cell Adhesion, Proliferation and Differentiation
3.4.1. Cell Adhesion (Short-Term Cultivation)
3.4.2. Cell Proliferation and Differentiation (Long-Term Cultivation)
Cell Proliferation (Static Cultivation)
Cell Differentiation (Dynamic Cultivation).
3.5. Phagocytosis of Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polymers. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Modjarrad, K.; Ebnesajjad, S.; Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable Polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; William Andrew Publishing: Chadds Ford, PA, USA, 2014; pp. 303–335. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable Polymers. In Principles of Tissue Engineering; Academic Press: Amsterdam, The Netherlands, 2014; pp. 441–473. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ye, M.; Park, K. Biodegradable polymers for microencapsulation of drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Langer, R. The evolution of biomaterials. Interview by Alison Stoddart and Victoria Cleave. Nat. Mater. 2009, 8, 444–445. [Google Scholar] [PubMed]
- Williams, D. The continuing evolution of biomaterials. Biomaterials 2011, 32, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and Biodegradable Nanocomposites and Hybrid Biomaterials for Bone Regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadpoor, A.A. The evolution of biomaterials research. Mater. Today 2013, 16, 408–409. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2016, 19, 2–3. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Guryanov, I.; Fiorucci, S.; Tennikova, T. Receptor-ligand interactions: Advanced biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Mooney, D.J. Cell instructive polymers. Adv. Biochem. Eng. Biotechnol. 2006, 102, 113–137. [Google Scholar] [PubMed]
- Stolnik, S.; Dunn, S.E.; Garnett, M.C.; Davies, M.C.; Coombes, A.G.A.; Taylor, D.C.; Irving, M.P.; Purkiss, S.C.; Tadros, T.F.; Davis, S.S.; et al. Surface Modification of Poly(lactide-co-glycolide) Nanospheres by Biodegradable Poly(lactide)-Poly(ethylene glycol) Copolymers. Pharm. Res. 1994, 11, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Mahmoud, A.A.; Kamel, R. A Novel Method for Preparing Surface-Modified Fluocinolone Acetonide Loaded PLGA Nanoparticles for Ocular Use: In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2015, 17, 1159–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punet, X.; Mauchauffe, R.; Giannotti, M.I.; Rodriguez-Cabello, J.C.; Sanz, F.; Engel, E.; Mateos-Timoneda, M.A.; Planell, J.A. Enhanced cell-material interactions through the biofunctionalization of polymeric surfaces with engineered peptides. Biomacromolecules 2013, 14, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.A.; Vlakh, E.G.; Tennikova, T.B. Polymers in orthopedic surgery and tissue engineering: From engineering materials to smart biofunctionalization of a surface. Polym. Sci. Ser. A 2012, 54, 585–601. [Google Scholar] [CrossRef]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of polycations: Relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Gombotz, H. Immobilization of Biomolecules and Cells on and within Synthetic Polymeric Hydrogels. Hydrogels Med. Pharm. 1986, 5, 246–247. [Google Scholar]
- Cass, T. Imobilized Biomolecules in Analysis: A Practical Approach; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science, Engineering and Technology; InTech: London, UK, 2006; pp. 247–282. [Google Scholar]
- Teske, M.; Rohm, H.W.; Kunna, K.; Keul, H.; Wilhelmi, M.; Jockenhövel, S.; Ovsianikov, A.; Schmitz, K.P.; Sternberg, K. Chemical surface modification of poly(ε-caprolactone) for accelerated wound healing after implantation of vascular devices. In World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 9–12 September 2009; Dössel, O., Schlegel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 215–218. [Google Scholar]
- Korzhikov-Vlakh, V.; Krylova, M.; Sinitsyna, E.; Ivankova, E.; Averianov, I.; Tennikova, T. Hydrogel Layers on the Surface of Polyester-Based Materials for Improvement of Their Biointeractions and Controlled Release of Proteins. Polymers 2016, 8, 418. [Google Scholar] [CrossRef]
- Kim, K.K.; Pack, D.W. Microspheres for Drug Delivery. Biol. Biomed. Nanotechnol. 2006, 19–50. [Google Scholar]
- Kim, T.G.; Park, T.G. Surface Functionalized Electrospun Biodegradable Nanofibersfor Immobilization of Bioactive Molecules. Biotechnol. Prog. 2006, 22, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhu, Z.; Hoye, T.R.; Macosko, C.W. Maleimide functionalized poly(ε-caprolactone)-block-poly(ethylene glycol) (PCL-PEG-MAL): Synthesis, nanoparticle formation, and thiol conjugation. Macromol. Chem. Phys. 2009, 210, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.G.; Waterhouse, A.; Kondyurin, A.; Bilek, M.M.; Weiss, A.S. Plasma-based biofunctionalization of vascular implants. Nanomedicine 2012, 7, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Krok-Borkowicz, M.; Musial, O.; Kruczala, P.; Dobrzynski, P.; Douglas, T.E.L.; Vlierberghe, S. Van; Dubruel, P.; Pamula, E. Biofunctionalization of poly(l-lactide-co-glycolide) by post-plasma grafting of 2-aminoethyl methacrylate and gelatin immobilization. Mater. Lett. 2015, 139, 344–347. [Google Scholar] [CrossRef]
- Manshor, M.R.; Anuar, H.; Nur Aimi, M.N.; Ahmad Fitrie, M.I.; Wan Nazri, W.B.; Sapuan, S.M.; El-Shekeil, Y.A.; Wahit, M.U. Mechanical, thermal and morphological properties of durian skin fibre reinforced PLA biocomposites. Mater. Des. 2014, 59, 279–286. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Herrero Acero, E.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(l-lactic acid) for drug delivery applications. Process Biochem. 2017, 59, 77–83. [Google Scholar] [CrossRef]
- Lee, S.H.; Yeo, S.Y. Improvement of hydrophilicity of polylactic acid (PLA) fabrics by means of a proteolytic enzyme from Bacillus licheniformis. Fibers Polym. 2016, 17, 1154–1161. [Google Scholar] [CrossRef]
- Gu, M.Q.; Yuan, X.B.; Kang, C.S.; Zhao, Y.H.; Tian, N.J.; Pu, P.Y.; Sheng, J. Surface biofunctionalization of PLA nanoparticles through amphiphilic polysaccharide coating and ligand coupling: Evaluation of biofunctionalization and drug releasing behavior. Carbohydr. Polym. 2007, 67, 417–426. [Google Scholar] [CrossRef]
- Zeng, S.; Ye, J.; Cui, Z.; Si, J.; Wang, Q.; Wang, X.; Peng, K.; Chen, W. Surface biofunctionalization of three-dimensional porous poly(lactic acid) scaffold using chitosan/OGP coating for bone tissue engineering. Mater. Sci. Eng. C 2017, 77, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ghotbi, Z.; Haddadi, A.; Hamdy, S.; Hung, R.W.; Samuel, J.; Lavasanifar, A. Active targeting of dendritic cells with mannan-decorated PLGA nanoparticles. J. Drug Target. 2011, 19, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, J.; Cao, L.; Yu, Y.; Liu, C. Enhanced osteogenesis of bone morphology protein-2 in 2-N,6-O-sulfated chitosan immobilized PLGA scaffolds. Colloids Surf. B Biointerfaces 2014, 122, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kurpinski, K.; Quigley, R.; Gao, H.; Hsiao, B.S.; Poo, M.M.; Li, S. Bioactive nanofibers: Synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007, 7, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.A.; Diederichs, S.; Nazarova, O. V; Vlakh, E.G.; Kasper, C.; Panarin, E.F.; Tennikova, T.B. Water-Soluble Aldehyde-Bearing Polymers of 2-Deoxy-2-methacrylamido-d-glucose for Bone Tissue Engineering. J. Appl. Polym. Sci. 2008, 108, 2386–2397. [Google Scholar] [CrossRef]
- Korzhikov, V.; Roeker, S.; Vlakh, E.; Kasper, C.; Tennikova, T. Synthesis of multifunctional polyvinylsaccharide containing controllable amounts of biospecific ligands. Bioconjug. Chem. 2008, 19, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Roeker, S.; Böhm, S.; Diederichs, S.; Bode, F.; Quade, A.; Korzhikov, V.; Van Griensven, M.; Tennikova, T.B.; Kasper, C. A study on the influence of biocompatible composites with bioactive ligands toward their effect on cell adhesion and growth for the application in bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Korzhikov, V.A.; Tennikova, T.B. Synthesis of poly(lactic acid) and the formation of poly(lactic acid)-based supraporous biofunctional materials for tissue engineering. Polym. Sci. Ser. B 2015, 57, 336–348. [Google Scholar] [CrossRef]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: the patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Kramm, D.E.; Kolb, C.L. Schiff Reagent Its Preparation and Its Use in the Determination of Formaldehyde in Cellulose Acetate Formal. Anal. Chem. 1955, 27, 1076–1079. [Google Scholar] [CrossRef]
- Tarnowski, B.I.; Spinale, F.G.; Nicholson, J.H. DAPI as a Useful Stain for Nuclear Quantitation. Biotech. Histochem. 1991, 66, 296–302. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Biodegradable Polymers; Lendlein, A.; Sisson, A. (Eds.) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Rothdiener, M.; Beuttler, J.; Messerschmidt, S.K.; Kontermann, R.E. Antibody targeting of nanoparticles to tumor-specific receptors: immunoliposomes. Methods Mol. Biol. 2010, 624, 295–308. [Google Scholar] [PubMed]
- Kazemi-Lomedasht, F.; Pooshang-Bagheri, K.; Habibi-Anbouhi, M.; Hajizadeh-Safar, E.; Shahbazzadeh, D.; Mirzahosseini, H.; Behdani, M. In vivo immunotherapy of lung cancer using cross-species reactive vascular endothelial growth factor nanobodies. Iran. J. Basic Med. Sci. 2017, 20, 489–496. [Google Scholar] [PubMed]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, Y.; Ikada, Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef]
- Pierigè, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef] [PubMed]












| Polyester | [NaOH], M | Sample Weight, mg | [COOH], nmol/mg (Titration) | [NH2], nmol/mg (Photometrical) |
|---|---|---|---|---|
| Matrices | ||||
| PLA | 0.1 | 52 | 4.3 ± 0.2 | 2.2 ± 0.1 |
| 1.0 | 47 | 5.1 ± 0.3 | 4.5 ± 0.2 | |
| PCL | 0.1 | 51 | 6.8 ± 0.4 | 7.2 ± 0.4 |
| 1.0 | 55 | 8.8 ± 0.5 | 8.1 ± 0.4 | |
| Particles | ||||
| PLA | 0.01 | 12 | 8.4 ± 0.4 | 6.5 ± 0.3 |
| 0.10 | 15 | 14.1 ± 0.7 | 11.0 ± 0.6 | |
| PCL | 0.01 | 10 | 6.0 ± 0.3 | 3.9 ± 0.2 |
| 0.10 | 13 | 10.3 ± 0.5 | 7.8 ± 0.4 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers 2018, 10, 1299. https://doi.org/10.3390/polym10121299
Korzhikov-Vlakh V, Averianov I, Sinitsyna E, Nashchekina Y, Polyakov D, Guryanov I, Lavrentieva A, Raddatz L, Korzhikova-Vlakh E, Scheper T, et al. Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers. 2018; 10(12):1299. https://doi.org/10.3390/polym10121299
Chicago/Turabian StyleKorzhikov-Vlakh, Viktor, Ilia Averianov, Ekaterina Sinitsyna, Yuliya Nashchekina, Dmitry Polyakov, Ivan Guryanov, Antonina Lavrentieva, Lukas Raddatz, Evgenia Korzhikova-Vlakh, Thomas Scheper, and et al. 2018. "Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces" Polymers 10, no. 12: 1299. https://doi.org/10.3390/polym10121299
APA StyleKorzhikov-Vlakh, V., Averianov, I., Sinitsyna, E., Nashchekina, Y., Polyakov, D., Guryanov, I., Lavrentieva, A., Raddatz, L., Korzhikova-Vlakh, E., Scheper, T., & Tennikova, T. (2018). Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers, 10(12), 1299. https://doi.org/10.3390/polym10121299