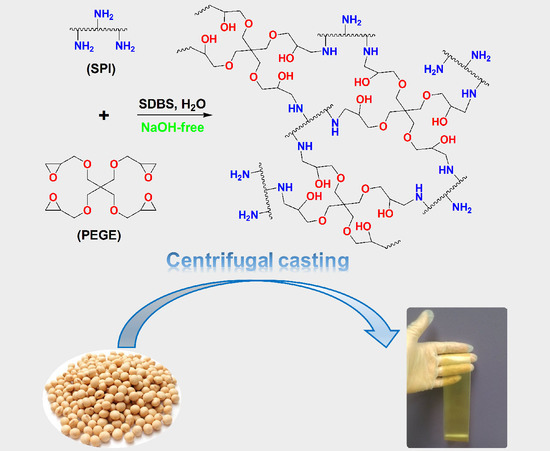
Sodium Hydroxide-Free Soy Protein Isolate-Based Films Crosslinked by Pentaerythritol Glycidyl Ether
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Casting
2.3. FTIR Analysis
2.4. XRD Analysis
2.5. Tensile Tests
2.6. SEM Observation
2.7. TGA Examination
2.8. Water Submersion Tests
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemically Crosslinking of Films
3.2. Thermal Stability of Films
3.3. Crystallinity of Films
3.4. Micromorphology Study of Films
3.5. Mechanical Properties of Films
3.6. Water Resistance of Films
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Avella, M.; Errico, M.E.; Rimedio, R.; Sadocco, P. Preparation of biodegradable polyesters/high-amylose-starch composites by reactive blending and their characterization. J. Appl. Polym. Sci. 2002, 83, 1432–1442. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Wu, Y.J.; Xia, C.L.; Cai, L.P.; Shi, S.Q.; Cheng, J.T. Water-resistant hemp fiber-reinforced composites: In-situ surface protection by polyethylene film. Ind. Crop Prod. 2018, 112, 210–216. [Google Scholar] [CrossRef]
- Wu, Y.J.; Xia, C.L.; Cai, L.P.; Garcia, A.C.; Shi, S.Q. Development of natural fiber-reinforced composite with comparable mechanical properties and reduced energy consumption and environmental impacts for replacing automotive glass-fiber sheet molding compound. J. Clean Prod. 2018, 184, 92–100. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J. Crop-based biodegradable packaging and its environmental implications. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–25. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Sridhar, R.; Immanuel, V.P. Development of model for mechanical properties of tapioca starch based edible films. Ind. Crop Prod. 2013, 42, 159–168. [Google Scholar] [CrossRef]
- Lodha, P.; Netravali, A.N. Thermal and mechanical properties of environment-friendly ‘green’ plastics from stearic acid modified-soy protein isolate. Ind. Crop Prod. 2005, 21, 49–64. [Google Scholar] [CrossRef]
- Ren, X.; Soucek, M. Soya-based coatings and adhesives. Soy-Based Chem. Mater. 2014, 1178, 207–254. [Google Scholar]
- Rhim, J.W.; Gennadios, A.; Weller, C.L.; Hanna, M.A. Sodium dodecyl sulfate treatment improves properties of cast films from soy protein isolate. Ind. Crop Prod. 2002, 15, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crop Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Liu, W.; Misra, M.; Askeland, P.; Drzal, L.T.; Mohanty, A.K. ‘Green’composites from soy based plastic and pineapple leaf fiber: Fabrication and properties evaluation. Polymer 2005, 46, 2710–2721. [Google Scholar] [CrossRef]
- Tummala, P.; Liu, W.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Influence of plasticizers on thermal and mechanical properties and morphology of soy-based bioplastics. Ind. Eng. Chem. Res. 2006, 45, 7491–7496. [Google Scholar] [CrossRef]
- Shi, S.Q.; Xia, C.; Cai, L. Modification of soy-based adhesives to enhance the bonding performance. In Bio-Based Wood Adhesives; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 96–120. [Google Scholar]
- Sui, C.; Zhang, W.; Ye, F.; Liu, X.; Yu, G. Preparation, physical, and mechanical properties of soy protein isolate/guar gum composite films prepared by solution casting. J. Appl. Polym. Sci. 2016, 133, 43382. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Shi, J.; Huang, X.; Peng, Q.; Xue, F. Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll. 2015, 45, 301–308. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Jiang, B.; Chen, J.; Jin, Z. Effect of the extent and morphology of phase separation on the thermal behavior of co-blending systems based on soy protein isolate/alginate. Food Hydrocoll. 2016, 52, 393–402. [Google Scholar] [CrossRef]
- Qiu, C.; Li, X.; Ji, N.; Qin, Y.; Sun, Q.; Xiong, L. Rheological properties and microstructure characterization of normal and waxy corn starch dry heated with soy protein isolate. Food Hydrocoll. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crop Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Han, Y.; Li, K.; Chen, H.; Li, J. Properties of Soy Protein Isolate Biopolymer Film Modified by Graphene. Polymers 2017, 9, 312. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Structure, functionality, and active release of nanoclay–soy protein films affected by clove essential oil. Food Bioprocess Technol. 2016, 9, 1937–1950. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crop Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- Denavi, G.A.; Pérez-Mateos, M.; Añón, M.C.; Montero, P.; Mauri, A.N.; Gomez-Guillen, M.C. Structural and functional properties of soy protein isolate and cod gelatin blend films. Food Hydrocoll. 2009, 23, 2094–2101. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Igarzabal, C.I.A. Study of Graft Copolymerization of Soy Protein-Methyl Methacrylate: Preparation and Characterization of Grafted Films. J. Polym. Environ. 2017, 25, 214–220. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, S.; Shi, J. Preparation and characterization of all-biomass soy protein isolate-based films enhanced by epoxy castor oil acid sodium and hydroxypropyl cellulose. Materials 2016, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Improvement of interfacial adhesion by bio-inspired catechol-functionalized soy protein with versatile reactivity: Preparation of fully utilizable soy-based film. Polymers 2017, 9, 95. [Google Scholar] [CrossRef]
- Jeevan Prasad Reddy, D.; Varada Rajulu, A.; Arumugam, V.; Naresh, M.; Muthukrishnan, M. Effects of resorcinol on the mechanical properties of soy protein isolate films. J. Plast. Film Sheet. 2009, 25, 221–233. [Google Scholar] [CrossRef]
- Foulk, J.A.; Bunn, J.M. Properties of compression-molded, acetylated soy protein films. Ind. Crop Prod. 2001, 14, 11–22. [Google Scholar] [CrossRef]
- Ghorpade, V.; Hanna, M. Mechanical properties of soy protein-polyethylene ribbon and film extrudates. Trans. ASAE 1996, 39, 611–615. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirviö, J.; Hormi, O.; Niinimäki, J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose 2013, 20, 741–749. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Marquié, C. Chemical reactions in cottonseed protein cross-linking by formaldehyde, glutaraldehyde, and glyoxal for the formation of protein films with enhanced mechanical properties. J. Agric. Food Chem. 2001, 49, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Iype, T.; Thomas, J.; Mohan, S.; Johnson, K.K.; George, L.E.; Ambattu, L.A.; Bhati, A.; Ailsworth, K.; Menon, B.; Rayabandla, S.M. A novel method for immobilization of proteins via entrapment of magnetic nanoparticles through epoxy cross-linking. Anal. Biochem. 2017, 519, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Physico-chemical properties improvement of soy protein isolate films through caffeic acid incorporation and tri-functional aziridine hybridization. Food Hydrocoll. 2016, 61, 923–932. [Google Scholar] [CrossRef]
- Xia, C.L.; Wang, L.; Dong, Y.M.; Zhang, S.F.; Shi, S.Q.; Cai, L.P.; Li, J.Z. Soy protein isolate-based films cross-linked by epoxidized soybean oil. RSC Adv. 2015, 5, 82765–82771. [Google Scholar] [CrossRef]
- Humer, E.; Zebeli, Q. Grains in ruminant feeding and potentials to enhance their nutritive and health value by chemical processing. Anim. Feed Sci. Technol. 2017, 226, 133–151. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo Cuenca, A.; Villanueva-Suárez, M.; Zapata-Revilla, M. Soybean, a promising health source. Nutr. Hosp. 2008, 23, 305–312. [Google Scholar] [PubMed]
- Ping, Y.; Chen, Z.; Ding, Q.; Zheng, Q.; Lin, Y.; Peng, Y. Ru-catalyzed ortho-oxidative alkenylation of 2-arylbenzo [d] thiazoles in aqueous solution of anionic surfactant sodium dodecylbenzenesulfonate (SDBS). Tetrahedron 2017, 73, 594–603. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Schmidt, V.; Giacomelli, C.; Soldi, V. Thermal stability of films formed by soy protein isolate–sodium dodecyl sulfate. Polym. Degrad. Stabil. 2005, 87, 25–31. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of eco-friendly soy protein isolate films with high mechanical properties through hnts, pva, and ptge synergism effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7S and 11S globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Duncan, O.D.; Duncan, B. A methodological analysis of segregation indexes. Am. Sociol. Rev. 1955, 20, 210–217. [Google Scholar] [CrossRef]
- Xia, C.L.; Zhang, S.F.; Shi, S.Q.; Cai, L.P.; Garcia, A.C.; Rizvi, H.R.; D’Souza, N.A. Property enhancement of soy protein isolate-based films by introducing POSS. Int. J. Biol. Macromol. 2016, 82, 168–173. [Google Scholar] [CrossRef] [PubMed]







| Film | SPI | DI-Water | Glycerol | SDBS | PEGE | |
|---|---|---|---|---|---|---|
| (g) | (mL) | (g) | (g) | (g) | (%) 1 | |
| SPI | 4 | 40 | 2 | - | - | - |
| SPI/SDBS | 4 | 40 | 2 | 0.8 | - | - |
| SPI/SDBS/PEGE1% | 4 | 40 | 2 | 0.8 | 0.04 | 1 |
| SPI/SDBS/PEGE2% | 4 | 40 | 2 | 0.8 | 0.08 | 2 |
| SPI/SDBS/PEGE4% | 4 | 40 | 2 | 0.8 | 0.16 | 4 |
| SPI/SDBS/PEGE6% | 4 | 40 | 2 | 0.8 | 0.24 | 6 |
| SPI/SDBS/PEGE8% | 4 | 40 | 2 | 0.8 | 0.32 | 8 |
| SPI/PEGE4% | 4 | 40 | 2 | - | 0.16 | 4 |
| Specimen | Tint1 1 | Tmax1 2 | Tint2 | Tmax2 | Tint3 | Tmax3 |
|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | |
| SPI powder | - | - | 265.3 | 309.8 | - | - |
| SDBS | - | - | - | - | 448.2 | 464.0 |
| SPI | 134.8 | 152.2 | 174.5 | 303.2 | - | - |
| SPI/SDBS | 135.8 | 163.3 | 188.0 | 310.9 | 459.4 | 480.5 |
| SPI/SDBS/PEGE4% | 135.2 | 162.2 | 185.7 | 316.9 | 465.6 | 483.5 |
| SPI/PEGE4% | 133.7 | 156.4 | 173.8 | 311.0 | - | - |
| Specimen | RCI1 |
|---|---|
| (%) | |
| SPI powder | 30.1 ± 0.7 2 |
| SDBS | 28.7 ± 0.4 |
| SPI | 29.5 ± 0.9 |
| SPI/SDBS | 31.9 ± 0.7 |
| SPI/SDBS/PEGE4% | 21.0 ± 0.4 |
| SPI/PEGE4% | 29.4 ± 0.7 |
| PEGE Content | Tensile Modulus | Tensile Strength | Elongation at Break |
|---|---|---|---|
| (%) | (MPa) | (MPa) | (%) |
| 0 | 15.4 ± 2.4 1 (A) 2 | 2.2 ± 0.3 (A) | 178.7 ± 23.1 (A) |
| 1 | 32.5 ± 7.0 (B) | 2.9 ± 0.5 (A) | 142.5 ± 35.5 (AB) |
| 2 | 56.6 ± 9.0 (C) | 4.3 ± 1.0 (B) | 129.7 ± 29.1 (BC) |
| 4 | 75.0 ± 7.5 (D) | 5.1 ± 0.3 (B) | 126.7 ± 12.1 (BC) |
| 6 | 57.4 ± 3.9 (C) | 5.0 ± 0.2 (B) | 97.2 ± 15.4 (CD) |
| 8 | 61.9 ± 4.4 (C) | 4.9 ± 0.5 (B) | 83.7 ± 16.2 (D) |
| Film | Moisture Content | 24-h Water Absorption | Total Soluble Matter |
|---|---|---|---|
| (%) | (%) | (%) | |
| SPI | 34.2 ± 2.6 1 | 109.7 ± 11.0 (B) 2 | 27.5 ± 0.5 (AB) |
| SPI/SDBS | 31.0 ± 1.3 | 194.6 ± 11.1 (A) | 27.6 ± 0.6 (AB) |
| SPI/SDBS/PEGE1% | 28.6 ± 1.7 | 112.9 ± 10.0 (B) | 30.1 ± 1.0 (A) |
| SPI/SDBS/PEGE2% | 26.7 ± 2.3 | 97.1 ± 10.2 (BC) | 28.3 ± 1.5 (AB) |
| SPI/SDBS/PEGE4% | 26.5 ± 2.0 | 96.9 ± 6.8 (BC) | 26.4 ± 1.6 (B) |
| SPI/SDBS/PEGE6% | 26.6 ± 2.0 | 67.8 ± 3.8 (BC) | 29.1 ± 1.2 (AB) |
| SPI/SDBS/PEGE8% | 23.4 ± 2.0 | 66.4 ± 3.8 (BC) | 28.6 ± 2.8 (AB) |
| SPI/PEGE4% | 29.5 ± 1.9 | 48.5 ± 3.1 (C) | 27.8 ± 1.4 (AB) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Cai, L.; Wang, C.; Mei, C.; Shi, S.Q. Sodium Hydroxide-Free Soy Protein Isolate-Based Films Crosslinked by Pentaerythritol Glycidyl Ether. Polymers 2018, 10, 1300. https://doi.org/10.3390/polym10121300
Wu Y, Cai L, Wang C, Mei C, Shi SQ. Sodium Hydroxide-Free Soy Protein Isolate-Based Films Crosslinked by Pentaerythritol Glycidyl Ether. Polymers. 2018; 10(12):1300. https://doi.org/10.3390/polym10121300
Chicago/Turabian StyleWu, Yingji, Liping Cai, Chen Wang, Changtong Mei, and Sheldon Q. Shi. 2018. "Sodium Hydroxide-Free Soy Protein Isolate-Based Films Crosslinked by Pentaerythritol Glycidyl Ether" Polymers 10, no. 12: 1300. https://doi.org/10.3390/polym10121300
APA StyleWu, Y., Cai, L., Wang, C., Mei, C., & Shi, S. Q. (2018). Sodium Hydroxide-Free Soy Protein Isolate-Based Films Crosslinked by Pentaerythritol Glycidyl Ether. Polymers, 10(12), 1300. https://doi.org/10.3390/polym10121300