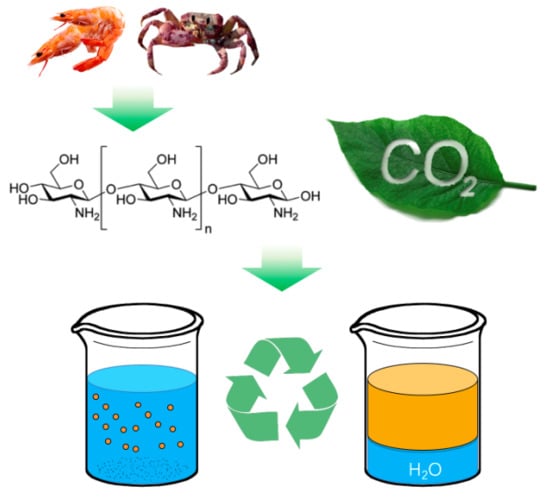
Reversible Stability of Emulsion and Polymer Latex Controlled by Oligochitosan and CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polystyrene Latex
2.3. Persistency Tests of Target Colloid Solutions Triggered by CO2
2.4. Characteristics
3. Results and Discussion
3.1. CO2-Switchable Properties of Oligochitosan
3.2. Reversible Emulsion
3.3. Switchable Salt Addition of Polystyrene Latex
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, G.; Yang, H.; Hou, Q.; Guo, D.; Chen, G.; Liu, F.; Chen, T.; Shi, X.; Su, Y.; Wang, J. A pH and salt dually responsive emulsion in the presence of amphiphilic macromolecules. J. Soft Matter 2018, 14, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Dube, G.P. Interaction between two hydrophobic colloidal particles, using the approximate Debye-Huckel theory. I. General properties. Trans. Faraday Soc. 1940, 35, 1125–1141. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2–triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Lin, S.; Theato, P. CO2–responsive polymers. Macromol. Rapid Commun. 2013, 34, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2–responsive polymeric materials: Synthesis, self–assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef]
- Vanderveen, J.R.; Durelle, J.; Jessop, P.G. Design and evaluation of switchable–hydrophilicity solvents. Green Chem. 2014, 16, 1187–1197. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Lu, Y.; Li, B.; Zhu, S. Reversibly coagulatable and redispersible polystyrene latex prepared by emulsion polymerization of styrene containing switchable amidine. Macromolecules 2011, 44, 6539–6545. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Wang, W.; Li, B.; Zhu, S. Preparation of CO2/N2-triggered reversibly coagulatable and redispersible polyacrylate latexes by emulsion polymerization using a polymeric surfactant. Macromol. Rapid Commun. 2012, 33, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, G.; Wang, W.; Yuan, H.; Li, B.; Zhu, S. Preparation of N2/CO2 triggered reversibly coagulatable and redispersible latexes by emulsion polymerization of styrene with a reactive switchable surfactant. Langmuir 2012, 28, 5940–5946. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tong, X.; Boissière, O.; Zhao, Y. General strategy for making CO2–switchable polymers. ACS Macro Lett. 2012, 1, 57–61. [Google Scholar] [CrossRef]
- Mercer, S.M.; Jessop, P.G. “Switchable Water”: Aqueous Solutions of Switchable Ionic Strength. ChemSusChem 2010, 3, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.; Harjani, J.R.; Phan, L.; Jessop, P.G.; Hodson, P.V. Effects–driven chemical design: The acute toxicity of CO2–triggered switchable surfactants to rainbow trout can be predicted from octanol–water partition coefficient. Green Chem. 2012, 14, 357–362. [Google Scholar] [CrossRef]
- Dostál, J. Two faces of alkaloids. J. Chem. Educ. 2000, 77, 993–998. [Google Scholar] [CrossRef]
- MacNaughton, J.; Moewes, A. Electronic structure of the nucleobases. J. Phys. Chem. B 2005, 109, 7749–7757. [Google Scholar] [CrossRef] [PubMed]
- Shu, C. Degradation products formed from oligochitosan in water. J. Agric. Food Chem. 1998, 46, 1129–1131. [Google Scholar] [CrossRef]
- Su, X.; Nishizawa, K.; Bultz, E.; Sawamoto, M.; Ouchi, M.; Jessop, P.G.; Cunningham, M.F. Living CO2–switchable latexes prepared via emulsion ATRP and AGET miniemulsion ATRP. Macromolecules 2016, 49, 6251–6259. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z. Correlating surface activity with structural and environmental parameters for alkylamidosulfobetaine surfactants. Colloid Polym. Sci. 2016, 294, 957–963. [Google Scholar] [CrossRef]
- Luo, X.; Yin, H.; Li, X.; Su, X.; Feng, Y. CO2–Triggered microreactions in liquid marbles. Chem. Commun. 2018, 54, 9119–9122. [Google Scholar] [CrossRef]
- Ko, Y.G.; Shin, S.S.; Choi, U.S. Primary, secondary, and tertiary amines for CO2 capture: Designing for mesoporous CO2 adsorbents. J. Colloid Interface Sci. 2011, 361, 594–602. [Google Scholar] [CrossRef]
- Mysels, K.J. Surface tension of solutions of pure sodium dodecyl sulfate. Langmuir 1986, 2, 423–428. [Google Scholar] [CrossRef]
- Firooz, A.; Chen, P. Impact of carbon dioxide on the surface tension of 1-hexanol aqueous solutions. Colloids Surf. A 2011, 392, 355–364. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, J.; Wang, J.; Wang, Y.; Guo, Z.; Yan, H. Salt effect on the complex formation between polyelectrolyte and oppositely charged surfactant in aqueous solution. J. Phys. Chem. B 2005, 109, 10807–10812. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, A.; Tomaiuolo, G.; Preziosi, V.; Guido, S. Emulsions in porous media: From single droplet behavior to applications for oil recovery. Adv. Colloid Interface Sci. 2018, 256, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, E.; Perazzo, A.; Arnold, C.B.; Stone, H.A. Salt type and concentration affect the viscoelasticity of polyelectrolyte solutions. Appl. Phys. Lett. 2018, 112, 203701. [Google Scholar] [CrossRef]
- Giudice, F.D.; Calcagno, V.; Taliento, V.E.; Greco, F.; Netti, P.A.; Maffettone, P.L. Relaxation time of polyelectrolyte solutions: When μ-rheometry steps in charge. J. Rheol. 2017, 61, 13–21. [Google Scholar] [CrossRef]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Guo, N.; Zhang, X.; Ou, W.; Yang, S.; Su, X.; Feng, Y. Reversible Stability of Emulsion and Polymer Latex Controlled by Oligochitosan and CO2. Polymers 2018, 10, 1352. https://doi.org/10.3390/polym10121352
Li L, Guo N, Zhang X, Ou W, Yang S, Su X, Feng Y. Reversible Stability of Emulsion and Polymer Latex Controlled by Oligochitosan and CO2. Polymers. 2018; 10(12):1352. https://doi.org/10.3390/polym10121352
Chicago/Turabian StyleLi, Liang, Na Guo, Xiao Zhang, Wen Ou, Shengcai Yang, Xin Su, and Yujun Feng. 2018. "Reversible Stability of Emulsion and Polymer Latex Controlled by Oligochitosan and CO2" Polymers 10, no. 12: 1352. https://doi.org/10.3390/polym10121352
APA StyleLi, L., Guo, N., Zhang, X., Ou, W., Yang, S., Su, X., & Feng, Y. (2018). Reversible Stability of Emulsion and Polymer Latex Controlled by Oligochitosan and CO2. Polymers, 10(12), 1352. https://doi.org/10.3390/polym10121352