When PEG is added to the RTIL/LiTFSI mixture, a η decrease is seen in RTILs with TFSI anion, i.e., those where the addition of LiTFSI had caused a very large η increase. This η decrease can be explained by the ability of PEG molecules to complex Li+, and “remove” it from the RTIL solution. Besides, in the PMPTFSI/LiTFSI/PEG mixture no crystalline phases are seen, which will make its viscosity lower than that of the PMPTFSI/LiTFSI mixture. In turn, the increase of η produced by the addition of PEG in the solutions of LiTFSI in RTILs with FSI is simply due to the higher η of pure PEG with respect to these RTIL/LiTFSI mixtures. This results in very similar η and η(T) for all the model phases regardless of the RTIL nature.
The study of the model liquid phases offers very interesting information. First, there is a decrease in Tc on adding LiTFSI to any of the RTILs. Second, as diffusivity is inversely proportional to η (if the Stokes–Einstein equation holds), then if the electrolytes with the different RTILs behave as the model phases with PEG, the ionic diffusivity will be similar in all of them, because η in the ternary mixtures varies little, only from 104 to 158 mPa·s at 25 °C. However, if the electrolytes with different RTILs behave like the model phases without PEG, the ionic diffusivity will be very different in all of them because η in the binary mixtures varies strongly, from 44 to 790 mPa·s at 25 °C. These two Δη limits mark the two limit morphologies of the electrolytes, from a complete mixing (which would resemble the ternary mixture behavior) to a complete phase separation (featured by the binary mixture).
3.1. Physicochemical Characterization of the Electrolytes
Figure 2a is a SEM image of EMIFSI-3c where abundant isolated TPGS-S can be seen. This is how TPGS-S appears in the electrolytes processed for 20 min and premixed. SEM imaging also indicates (not shown) that few or no crystalline morphologies are detected in these electrolytes, contrary to what happened in electrolytes with less [RTIL] [
16], where morphologies related to crystalline order were seen in all electrolytes except those prepared with EMIFSI. In
Figure 2b the FTIR spectra of the υ(S-N) region of FSI and TFSI in several electrolytes appear. This vibration is related to the aggregation state of the anions [
22]. In the lower half, the same region is shown for the pure compounds EMIFSI, LiTFSI, EMITFSI, and LiFSI. The electrolytes that contain only the TFSI anion show a spectrum almost identical to that of TFSI as in EMITFSI (mild interaction of TFSI with the cation, either EMI or PMP), and the bands corresponding to TFSI, as in LiTFSI (strong interaction between TFSI and the cation), are not seen. This is because, when LiTFSI is added to the mixture of ionic liquid and PEO, Li becomes preferentially coordinated by the PEO chain, leaving TFSI free to become involved in milder interactions with the cations of the ionic liquid. In the electrolytes with a mixture of FSI and TFSI anions, the spectral region is broad and a mixture of species corresponding to TFSI as in EMITFSI (739 cm
−1) and FSI as in EMIFSI (or PMPFSI) can be suspected. Again, Li is dissolved by the PEO chain, and the anions TFSI and FSI interact with the ionic liquid cation (EMI or PMP). These spectra suggest that the liquid phase in the electrolytes is closer to the model liquid phases prepared with PEG than to those without PEG (see
Figure 1), and so no ionic liquid crystallization is to be expected. Moreover, no extreme differences in viscosity are to be expected either.
In
Figure 2c the DSC scans of the electrolytes are collected; in general they show complex multiphasic materials. Electrolytes with EMIFSI liquid tend to be less crystalline than analogue electrolytes with the other RTILs. The PEO melting endotherm, which, when pure, appears close to 60 °C, and involves over 60 wt % of the polymer, is well seen in PMPFSI-4, PMPFSI-6, EMITFSI-1, and all PMPTFSI between 40 and 60 °C, but it is imperceptible or almost so in EMIFSI-3c, EMIFSI-3f, EMIFSI-4, and EMITFSI-2. Compare, for example, the χ
c of EMIFSI-2 with PMPFSI-1, PMPFSI-2, or PMPFSI-3.
Table 2 also shows that the higher the [RTIL], the lower the χ
c (irrespective of the RTIL).
In some of the electrolytes, especially those prepared with EMIFSI, it is possible to see a small melting endotherm at T > 60 °C (PEO melting temperature), ranging from 75 to 100 °C. This endotherm is more conspicuous in the first scanning than in subsequent ones, as if this crystalline phase requires time to develop. It is very clear in EMIFSI-1 (which is the most crystalline electrolyte among those prepared with EMIFSI), but exists in most of the electrolytes prepared with EMIFSI, at least on the first scan. It is also seen in three electrolytes prepared with PMPFSI, PMPFSI-1, PMPFSI-3, and PMPFSI-5, and does not appear in the electrolytes prepared with PMPTFSI or EMITFSI. This suggests that this high T melting peak is related to a phase containing PEO and FSI.
As for the low-temperature region,
Figure 2c shows that many of the electrolytes seem to be one or several
Tg under that of pure PEO, even at
T < −60 °C. This is caused by the interaction of the salt LiTFSI and the RTIL with PEO, and reveals that there is compatibility among the components. It is known that Li salts and LiTFSI in particular plasticize the PEO chain [
23]. In those with the largest concentration of RTIL + LiTFSI, however, no
Tg is seen. The exception is EMIFSI-3g, which has a very short residence time in the extruder, where the PEO
Tg is seen at about −41 °C, suggesting the existence of a PEO-rich phase.
Given the complexity of the composition (at least three and usually four components in each electrolyte) and the multiphasic character of some of the components (PEO has a Tm ≈ 60 °C and Tg ≈ −40 °C, the RTILs have Tm between −16 °C and 12 °C) it is not surprising that their blends display numerous phases. As explained before in relation to the model liquid phases, the final heterogeneity of the electrolytes will depend on how compatible the components are and how complete the mixing has been. Considering the basic features of polymer blending, the well-known unlikeliness of thermodynamic mixing, and their high viscosity, the quality of mixing and hence the final morphology of these electrolytes depends very strongly on the processing conditions. This is relevant in these materials due to their ultimate purpose of becoming industrially scalable electrolytes.
To summarize, these electrolytes are little crystalline, especially the EMIFSI-containing ones, and display a complex phase distribution in the T range −80 °C to 100 °C that is strongly dependent on the mixing quality, i.e., on processing conditions. SEM imaging shows good dispersion of the TPGS-S fibers and FTIR reveals that in all of the electrolytes, complexation of Li by the PEO chain occurs extensively.
3.2. Rheology
In an attempt to reduce the solid phases in the electrolytes (in favor of liquid ones), the amount of TPGS-S has been reduced from 5 wt % [
16] to 2.5% in some of the electrolytes. Additionally, the effect of sepiolite organic modification has been checked by blending a sample with pure sepiolite (EMIFSI-3d) instead of TPGS-S. Then, a rheological study was done, which is summarized in
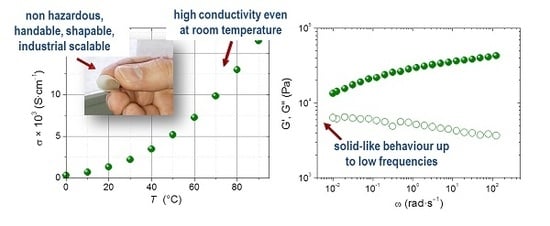
Figure 3, where the effect of [RTIL] and processing conditions is studied. The effect of TPGS-S instead of pure sepiolite S is illustrated with a simple creep experiment, described in detail in the experimental section, which basically consists of placing the electrolytes between the electrodes to endure 0.5 kg for 20 min at 75 °C and then at 90 °C. After this experiment, EMIFSI-3d, which contains pure sepiolite, showed signs of creep, while the other samples did not. Pictures showing the appearance of the sandwiched electrolytes, EMIFSI-3d, EMIFSI-3f, and EMIFSI-3g, after this creep test are shown in
Figure 3a. This is noteworthy as EMIFSI-3f and EMIFSI-3g contain only 2.5 wt % of TPGS-S, and EMIFSI-3g has been processed for only 4 min, instead of 20 min as with the rest of the samples. This different processing means the mixing is not so homogeneous (as shown in the DSC) but, interestingly, EMIFSI-3g is still solid-like. From a practical viewpoint, and having industrial scalability in mind, the fact that solid-like electrolytes can be made by melt-compounding in such a simple and quick way is highly interesting.
In columns 4 and 5 of
Table 2, the values of G′ at 75 °C and 0.05 rad·s
−1 and the frequency at which G′ = G″ are collected. A clear effect of premixing on the solid character of the samples is seen. We see that all non-premixed electrolytes (irrespective of nature and concentration of the RTIL) show lower G′ values and crossover (G′ = G″) frequencies ω > 0.01 rad·s
−1, while all premixed samples display no crossover up to ω = 0.01 rad·s
−1 and have G′ values over 2-fold higher (even EMIFSI-3g with very low extrusion time). This is certainly caused by the different quality of the TPGS-S distribution in the electrolytes, differences that are not detectable in SEM imaging. Premixing is, as regards rheology, the key to solid-like behavior.
In the pre-mixed EMIFSI-3n series, an additional creep experiment was done consisting of withstanding 0.1 kg at RT for 10 months. In none of the electrolytes tested was creep noticeable.
Figure 3a includes the picture of EMIFSI-3e after this test. The rheological measurements provide quantitative evidence of this pseudosolid behavior.
Figure 3b shows the shear storage (G′) and loss (G″) moduli variation with frequency at 75 °C for the set of pre-mixed electrolytes with the highest [RTIL]. EMIFSI-3d with pure sepiolite instead of TPGS-S has a lower G″ and G′ moduli than the other three samples. For ω < 0.02 rad·s
−1 G″ > G′, i.e., for the lower frequencies, EMIFSI-3d is behaving like a liquid. This does not occur for the electrolytes containing TPGS-S, EMIFSI-3c, EMIFSI-3f, and EMIFSI-3g, which present a higher G′ than G″ in the whole frequency range; most importantly, no crossover is seen, confirming their solid behavior at 75 °C.
3.3. Ion Conductivity and Diffusivity
Table 2 contains σ of the different electrolytes at 25 and 70 °C, and
Figure 4 represents the variation of σ with temperature in the range −50 °C to 90 °C of a selection of electrolytes. It is remarkable that, though the DSC in
Figure 2 shows the phase heterogeneity of the electrolytes, this has, as a rule, little effect on σ, as seen for instance in EMIFSI-3c, EMIFSI-3f, and EMIFSI-3g in
Figure 4a—all very similar as regards σ(T) in spite of the different low T phase distribution evidenced in their DSC. Another indication that phase transitions or relaxations are not being reflected in σ(T) is that the σ measurements performed on cooling from 90 °C or on heating from −80 °C coincide absolutely. Moreover, all electrolytes in
Figure 4a can be fitted with a single Vogel–Fulcher–Tamman (VFT) equation,
(Equation (1)), where σ
∞ (S·cm
−1), B (K), and T
0 are constants. The fittings are very good, indicating that the σ(T) dependence is characteristic of a viscous liquid in which σ is governed by η in the whole temperature range, and the VFT fitting of σ(T) reflects the VFT variation of η(T). The fitting parameters appear in
Table 4. Minor variations are obtained for T
0 and B parameters, related to the activation energy in the samples EMIFSI-3n, indicating that the different processing conditions employed in these electrolytes do not affect σ(T). The main differences between electrolytes with EMIFSI/LiTFSI and PMPFSI/LiTFSI are σ
∞, with the latter being less conductive at high temperatures, which is consistent with the lower η of EMIFSI and its mixtures in
Table 3. The fitting of EMIFSI-4 σ results in a set of different parameters because this electrolyte has no Li salt.
Almost all the electrolytes in
Table 2 behave like those in
Figure 4a, in the sense that no phase transitions are seen to affect σ(T). The exceptions are EMITFSI-1and PMPFSI-6, both without Li salt and a large fraction of RTIL. PMPFSI-6 appears in
Figure 4b: σ measured on heating and on cooling do not coincide, being slightly higher when measuring on cooling from the melt. In PMPFSI-6 σ does not follow a VFT decrease with T, but decreases very quickly at about 40 °C and −10 °C. The PMPFSI-6 DSC scan in the inset of
Figure 4b has two strong melting endotherms, one at about 40 °C and another at about −10 °C. PMPFSI-6 is crystalline in about a 20%. The transition at 40 °C is the melting of the PEO crystallites, while that at 10 °C is the PMPFSI melting (
Table 3). Thus, phase transitions involving large fractions of the electrolyte do appear as variations in σ(T).
Besides EMITFSI-1 and PMPFSI-6, there is a third electrolyte prepared without Li salt and a large [RTIL], which is EMIFSI-4. Contrary to EMITFSI-1 and PMPFSI-6, the σ(T) of EMIFSI-4 can be fitted with a VFT equation in the whole T range (see VFT parameters in
Table 4, suggesting that no phase transitions are occurring. It seems then that when the RTIL is EMIFSI, PEO finds it more difficult to crystallize, probably because of a larger interaction between the polymer chain and the RTIL.
Figure 5 shows the representation of σ as a function of [RTIL] under the PEO melting point, at 25 °C (in
Figure 5a), and above it, at 70 °C (
Figure 5b). Even if phase transitions are too mild to be detected in the σ(T) curves of
Figure 4, at 25 °C the presence of a certain amount of crystallinity divides the electrolytes into two groups, ones with χ
c < 15% (more conductive for the same [RTIL]), and ones with χ
c > 20%, which are less conductive. The first group comprises the electrolytes prepared with EMIFSI, EMITFSI-2, and most of those prepared with PMPTFSI, while the second group comprises most of the electrolytes prepared with PMPFSI, EMITFSI-1, and PMPTFSI-3. The most conductive electrolytes at 25 °C reach values close to 2 × 10
−3 S·cm
−1, remarkable for a solid electrolyte, and about 4-fold higher than those reported previously in similar electrolytes with lower [RTIL] [
16]. Of course, at 70 °C, when most of the crystallites have melted, the difference between the two groups disappears and though scatter is important (and expectable because of the different formulations) there is a very clear trend with the [RTIL], which is the most important factor in their overall σ. At 70 °C the solid electrolytes reach a remarkable σ ≈ 0.01 S·cm
−1.
The fact that the RTIL concentration is more determinant than the RTIL nature as regards the σ values seems to suggest that the electrolytes are behaving like the liquid model phases including PEG, rather than those model liquid phases not including PEG, as if the latter was the case very large variations of σ (caused by very large variations of η, see
Figure 1) would be observed and all σ would be clearly lower. That the electrolytes behave more like the PEG-containing model liquid phases is not surprising, and also suggests that Li is trapped in the PEO chain rather than moving solvated by FSI or TFSI anions, in accordance with the FTIR results in
Figure 2b.
Figure 5b shows how different processing conditions (EMIFSI-3b to EMIFSI-3g) produce a certain scatter in σ. Electrolytes EMIFSI-3a, 3b, and 3e, where no premixing was done, have slightly lower σ than EMIFSI-3c, 3d, 3f, and 3g, in which premixing was done. Those values obtained in electrolytes that were not premixed are closer to the line that fits all the electrolytes, where no premixing was done, which is very reasonable. Those that were premixed show more reproducible and slightly higher values of σ. In any case, values differ by less than 15%. This small variation is probably caused by the larger tortuosity of the electrolytes in which mixing has been less efficient. For polymer-based electrolytes such as those studied in this work, which we hope will become a real solution at an industrial scale, this scarce effect of different processing conditions in remarkable σ at and over room temperature is a most important issue, for multiphasic systems highly sensitive to processing conditions are very difficult to scale up.
Insight into the ionic transport characteristics of these electrolytes at a microscopic level is provided by the diffusion coefficients (D), which appear in
Table 2. The diffusivity of the anions is outstanding, bearing in mind that they are solid materials up to
T > 75 °C. In effect, D
TFSI or D
FSI of about 10
−11 m
2·s
−1 at 25 °C are closer to a liquid’s than to a solid’s transport behavior. This is not surprising for many of the electrolytes have
Tg < −40 °C and almost no crystallinity (
Table 2), and as a consequence they are liquids at a microscopic level. The fact that they behave as solids up to
T > 75 °C accounts for the excellent performance of TPGS-S as a physical cross-linker.
Making use of the data in
Table 2 and the Nernst–Einstein equation (Equation (2)),
, where n
i and D
i are the molar concentrations of the ion and its diffusion coefficient, respectively, it is possible to calculate the maximum conductivity if all the dissociation coefficients α
i were 1, which we have called σ
NE (column 8 of
Table 2). It has not been possible to measure the cation in the RTIL by NMR and so it has been estimated, according to the literature [
24], to be about 10% higher than FSI in the case of EMI and 10% lower in the case of PMP. The contribution of Li
+ to σ
NE, σ
NE(Li) has also been calculated and included in column 7 of
Table 2.
For a simple visualization of the differences between experimental and calculated σ, see
Figure 6a. Though scatter is important (R = 0.98), a linear relationship is still seen up to σ ≈ 1.2 × 10
−3 S·cm
−1, the slope of which is
m = 1.15, meaning that the average dissociation coefficient or ionicity of the electrolytes, α, which is the inverse of the slope, is about 0.87. This includes the LiTFSI salt dissociation and that of the RTILs employed. Pure RTILs such as those employed in this work have α in the range 0.5 to 0.7 at 25 °C, and LiTFSI is highly dissociated in the presence of PEO; this calculated value of α, though very high, is reasonable. Over σ ≈ 1.2 × 10
−3 S·cm
−1 a second linear relationship exists, with a higher slope, i.e., apparently lower overall ionicity. The electrolytes involved in this second trend all have the same formulation (with the highest [RTIL] used in this work) and differ only in the processing conditions, for they are the EMIFSI-3n set.
Figure 6b shows the variation of the three diffusion coefficients D
Li, D
TFSI, and D
FSI, as a function of σ. This illustrates how the contribution of Li to σ is much lower than that of the anions; as σ increases, it becomes progressively lower since the slope of D
Li is smaller than that of the anions. The increase of RTIL concentration makes the anions diffuse more quickly, to a much higher extent than Li
+. This agrees with the preferential motion of Li
+ in the PEO chain.
A single linear correlation is seen between σ and D
Li and D
TFSI for all the electrolytes measured, but a strong positive deviation is seen in the D
FSI of the EMIFSI-3n set. This explains the existence of a second linear relationship with a higher slope between σ
NE and σ in
Figure 6a, because the large D
FSI that makes σ
NE increase is not contributing to the experimental σ. However, the reason why D
FSI is increasing in such a way in the EMIFSI-3n is not straightforward.
The relationship between the diffusion coefficients appears in
Figure 7. In
Figure 7a, D
TFSI vs. D
FSI is represented. A straight line can be fitted irrespective of the type of ionic liquid, salt concentration, amount of sepiolite, etc., the slope of which is the ratio
, if the Stokes–Einstein equation holds (Equation (3)):
, where k is the Boltzmann constant (1.38 × 10
−23 J·K
−1), T is the absolute temperature, c is a constant with a proposed value of 5 [
25], η is the liquid viscosity, and r is the effective Stokes radius. Then,
, a value frequently found in electrolytes containing a high concentration of these anions [
26].
Figure 7b,c show the relationship between D
Li and D
TFSI or D
FSI, respectively. Those prepared with EMIFSI fit a line with a similar slope but a slightly higher intercept than the rest of the electrolytes. As a consequence, D
Li is always slightly higher for the same D
TFSI in EMIFSI electrolytes. This seems not to have important practical consequences, because though systematic the difference is small. That the slope is very similar (
m ≈ 0.1 for D
Li vs. D
TFSI, and
m = 0.07 for D
Li vs. D
FSI) implies that
and
are roughly the same, independent of the ionic liquid used;
and
. This very high
as compared to the anions’ is caused by the complexation of Li by the oxyethylenic units and its transport along the PEO chain. That Li moves mostly through the PEO chain provides an explanation for the slightly higher values of D
Li in EMIFSI electrolytes, as, being PEO less crystalline and better mixed in them, Li’s transport along the polymer chain is less tortuous.
Figure 8 shows the relationship of the electrolytes’
Tg with D
Li and D
TFSI. Only electrolytes in which a defined
Tg is observed in DSC traces (see
Table 2) have been used to prepare this graph. It is noteworthy that for D
TFSI a single dependence with
Tg is found irrespective of the RTIL nature, resembling what was shown in
Figure 5b, where it was evidenced that σ depends more on the [RTIL] than on its nature. It is also remarkable that a shift of 15 °C in
Tg makes D
TFSI vary by about 3-fold. Note that PMPTFSI-3, for which D
TFSI is not in the general trend, is much more crystalline than the rest of the electrolytes made with PMPTFSI, probably because of defective mixing. As for D
Li, it is surprising that even though evidence that Li is moving through the PEO chain is seen along the work, its value seems to be independent of the
Tg values.
The complex blends presented in this work exemplify the extraordinary potential of polymer-based materials as electrolytes because of their unique combination of electrical properties, excellent mechanical properties, sustainability, and simple scalability. Especially remarkable is the diffusivity of the anions FSI and TFSI at 25 °C, some of them even over 10−11 m2·s−1, which evidences high potential for efficient anion transport..