Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
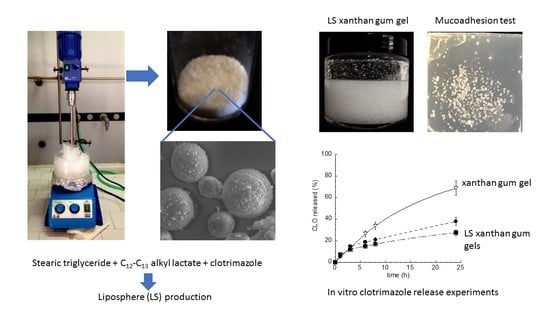
2.2.1. Liposphere Production
2.2.2. LS Morphological and Dimensional Analysis
2.2.3. CLO Content of LS
2.2.4. Anticandidal Activity Study
2.2.5. Gel Production
2.2.6. Viscosity Test
2.2.7. Spreadability Test
2.2.8. Gel Leakage and Adhesion Test
2.2.9. In Vitro CLO Release Studies
3. Results
3.1. Liposphere Production and Characterization
3.2. Anticandidal Activity Study
3.3. Production and Characterization of Liposphere Gels
3.3.1. Gel Viscosity
3.3.2. Gel Spreadability
3.3.3. Gel Leakage
3.3.4. Gel Adhesion
3.4. In Vitro CLO Release Kinetics
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LS | Liposphere |
| AL | C12-C13 alkyl lactate |
| TRIST | stearic triglyceride |
| TRIC | caprylic/capric trigliceride |
| CLO | clotrimazole |
| VPSEM | variable-pressure scanning electron microscopy |
| MIC | minimal inhibitory concentration |
References
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front. Immunol. 2017, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J. Vaginal candidosis: Epidemiological and etiological factors. Int. J. Gynecol. Obstet. 2000, 71, S21–S27. [Google Scholar] [CrossRef]
- Zhu, W.; Filler, S.G. Interactions of Candida albicans with epithelial cells. Cell. Microbiol. 2010, 12, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ravani, L.; Esposito, E.; Bories, C.; Moal, V.L.; Loiseau, P.M.; Djabourov, M.; Cortesi, R.; Bouchemal, K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013, 454, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kyle, A.A.; Dahl, M.V. Topical therapy for fungal infections. Am. J. Clin. Dermatol. 2004, 5, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.O.; Parente, M.E.; Ares, G. Brazilian Screening of mucoadhesive vaginal gel formulations. J. Pharm. Sci. 2014, 50, 931–941. [Google Scholar]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Lorenzoni, A.; Ferreira, L.M.; Mattiazzi, J.; Adams, A.I.H.; Denardi, L.B.; Sydney, H.A.; Schaffazick, S.R.; Cruz, L. Clotrimazole-loaded Eudragit® RS100 nanocapsules: Preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater. Sci. Eng. C 2013, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Esposito, E.; Luca, G.; Nastruzzi, C. Production of lipospheres as carriers for bioactive compounds. Biomaterials 2002, 23, 2283–2294. [Google Scholar] [CrossRef]
- Domb, A. Lipospheres for controlled delivery of substances. In Microencapsulation; Methods and Industrial Applications, 2nd ed.; Benita, S., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 297–316. [Google Scholar]
- Maniar, M.H.; Amselem, D.; Xie, S.; Burch, X.; Domb, R.A.J. Characterization of lipospheres: Effect of carrier and phospholipid on the loading of drug into the lipospheres. Pharm. Res. 1991, 8, 175–185. [Google Scholar]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Lipospheres and pro-nano lipospheres for delivery of poorly water soluble compounds. Chem. Phys. Lipids 2012, 165, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Dudala, T.B.; Yalavarthi, P.R.; Vadlamudi, H.C.; Thanniru, J.; Yaga, G.; Mudumala, N.L.; Pasupati, V.K. A perspective overview on lipospheres as lipid carrier systems. Int. J. Pharm. Investig. 2014, 4, 149–155. [Google Scholar] [PubMed]
- Barakat, N.S.; Yassin, A.E.B. In vitro characterization of carbamazipine-loaded precifac lipospheres. Drug Deliv. 2006, 13, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, H.N.; Patel, P.B.; Desai, B.G.; Ashok, P.; Arulmozhi, S. Design and statistical optimization of glipizide loaded lipospheres using response surface methodology. Acta Pharm. 2007, 57, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Haller, I. Mode of action of clotrimazole: Implications for therapy. Am. J. Obstet. Gynecol. 1985, 152, 939–944. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Contado, C.; Costenaro, A.; Drechsler, M.; Rossi, D.; Menegatti, E.; Sacchetti, G.; Cortesi, R. Clotrimazole nanoparticle gel for mucosal administration. Mater. Sci. Eng. C Mater. 2013, 33, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Production of Lipospheres for Bioactive Compound Delivery. In Lipospheres in Drug Targets and Delivery; Nastruzzi, C., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2005; pp. 23–40. [Google Scholar]
- Jenning, V.; Thünemann, A.; Gohla, S. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Lind, H.; Jonsson, H.; Schnqrer, J. Antifungal effect of dairy propionibacteria—Contribution of organic acids. Int. J. Food Microbiol. 2005, 98, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, S. Polysaccharides in Medicinal Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Denise, F.S.; Petri, J. Xanthan gum: A versatile biopolymer for biomedical and technological applications. Appl. Polym. Sci. 2015, 132, 42035–42048. [Google Scholar]
- Lazzari, A.; Kleinebudde, P.; Knop, K. Xanthan gum as a rate-controlling polymer for the development of alcohol resistant matrix tablets and mini-tablets. Int. J. Pharm. 2018, 536, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Gandhi, A.; Sen, K.K.; Basu, S.K. Natural polymers and their application in drug delivery and biomedical field. J. PharmaSciTech 2011, 1, 16–27. [Google Scholar]
- Griffin, B.J. Variable pressure and environmental scanning electron microscopy: Imaging of biological samples. Methods Mol. Biol. 2007, 369, 467–495. [Google Scholar] [PubMed]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Approved Standard, NCCLS Document M27-A2, No. 15; Clinical and Laboratory Standards Institute: Villanova, PA, USA, 2002; Volume 22. [Google Scholar]
- Jahn, B.; Martin, E.; Stueben, A.; Bhakdi, S. Susceptibility Testing of Candida albicans and Aspergillus Species by a Simple Microtiter Menadione-Augmented 3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2H-Tetrazolium Bromide Assay. J. Clin. Microbiol. 1995, 33, 661–667. [Google Scholar] [PubMed]
- Kaur, L.P.; Garg, R.; Gupta, G.D. Development and evaluation of topical gel of minoxidil from different polymer bases in application of alopecia. Int. J. Pharm. Pharm. Sci. 2010, 2, 43–47. [Google Scholar]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion-based vaginal gel of clotrimazole: Formulation, in vitro evaluation, and stability studies. AAPS PharmSciTech 2009, 10, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Lakshmanan, P. Effect of processing variables on characterization of ofloxacin loaded lipospheres prepared by melt dispersion technique. Curr. Drug Deliv. 2013, 10, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bennett, A.E. Synthesis, toxicology and potential of ordered mesoporous materials in nanomedicine. Nanomedicine 2001, 6, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Murgia, S.; Falchi, A.M.; Mano, M.; Lampis, S.; Angius, R.; Carnerup, A.M.; Schmidt, J.; Diaz, G.; Giacca, M.; Talmon, Y.; et al. Nanoparticles from Lipid-Based Liquid Crystals: Emulsifier Influence on Morphology and Cytotoxicity. J. Phys. Chem. B 2010, 114, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Karadzovska, D.; Brooks, J.D.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013, 65, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Han, C.D. Rheology and Processing of Polymeric Materials. Volume 1: Polymer Rheology; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of Semisolid Formulations. An Update. Pharm. Technol. 2002, 26, 84–105. [Google Scholar]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Lee, M.L.; Onderdonk, A.B. Effect of Candida albicans infection and clotrimazole treatment on vaginal microflora in vitro. Obstet. Gynecol. 1995, 86, 925–930. [Google Scholar] [CrossRef]
- Cosmacol Eli Data Sheet; Sasol Germany, GmbH: Hamburg, Germany, 2014.
- Iqbal, M.A.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carrier system: Recent advances in drug delivery. J. Drug Target 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Lajean Chaffin, W.; López-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martínez, J.P. Cell Wall and Secreted Proteins of Candida albicans: Identification, Function, and Expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar]
- Pendrak, M.L.; Klotz, S.A. Adherence of Candida albicans to host cells FEMS Microbiol. Lett. 1995, 129, 103–113. [Google Scholar]
- Das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.N.; Jadhav, J.K.; Masirkar, V.J.; Sakarkar, D.M. Formulation, optimization and evaluation of controlled release alginate microspheres using synergy gum blends. Res. J. Pharm. Technol. 2009, 2, 324–327. [Google Scholar]
- Xue, D.; Sethi, R. Viscoelastic gels of guar and xanthan gum mixtures provide long-term stabilization of iron micro- and nanoparticles. J. Nanopart. Res. 2012, 14, 1239–1258. [Google Scholar] [CrossRef]
- Dalla Vecchia, E.; Luna, M.; Sethi, R. Transport in porous media of highly concentrated iron micro- and nanoparticles in the presence of xanthan gum. Environ. Sci. Technol. 2009, 43, 8942–8947. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.J.; Alam, M.M.A.; Iqubal, Z.I.; Khar, R.K.; Ali, M. Development and in vitro evaluation of an acid buffering bioadhesive vaginal gel for mixed vaginal infections. Acta Pharm. 2008, 58, 407–419. [Google Scholar] [CrossRef] [PubMed]






| Formulation | Composition (% w/w) | |||||
|---|---|---|---|---|---|---|
| Tristearin (TRIST) | Caprylic/Capric Triglyceride (TRIC) | Alkyl Lactate (AL) | Clotrimazole (CLO) | |||
| LSTRIST | 100.00 | - | - | |||
| LSTRIST/TRIC | 70.00 | 30.00 | - | - | ||
| LSTRIST/AL30 | 70.00 | - | 30.00 | - | ||
| LSTRIST/AL15 | 85.00 | - | 15.00 | - | ||
| LSTRIST/AL10 | 90.00 | - | 10.00 | - | ||
| LSTRIST/AL1 | 99.00 | - | 1.00 | - | ||
| LSTRIST-CLO | 98.04 | - | 1.96 | |||
| LSTRIST/TRIC-CLO | 68.69 | 29.35 | - | 1.96 | ||
| LSTRIST/AL30-CLO | 68.69 | 29.35 | - | 1.96 | ||
| LSTRIST/AL15-CLO | 83.34 | - | 14.70 | 1.96 | ||
| LSTRIST/AL10-CLO | 88.24 | - | 9.80 | 1.96 | ||
| LSTRIST/AL1-CLO | 97.06 | - | 0.98 | 1.96 | ||
| Formulation | Gel Components (% w/w) | ||||
|---|---|---|---|---|---|
| Tristearin | Alkyl Lactate | Clotrimazole | Xanthan Gum | Water | |
| Gel LSTRIST-CLO | 4.902 | - | 0.098 | 0.500 | 94.500 |
| Gel LSTRIST/AL1-CLO | 4.853 | 0.049 | 0.098 | 0.500 | 94.500 |
| Gel-CLO | - | - | 0.098 | 0.500 | 99.402 |
| GelAL1-CLO | - | 0.049 | 0.098 | 0.500 | 99.353 |
| Formulation | Mean Diameter (μm) | Yield (%) a | CLO EE (%) b |
|---|---|---|---|
| LSTRIST | 50 ± 28 | 92.0 ± 1 | - |
| LSTRIST/TRIC | 6.3 ± 8 | 88.3 ± 2 | - |
| LSTRIST/AL30 | n.d. * | 80.0 ± 1 | - |
| LSTRIST/AL15 | n.d. * | 86.0 ± 2 | - |
| LSTRIST/AL10 | n.d. * | 89.7 ± 3 | - |
| LSTRIST/AL1 | 54.2 ± 30 | 93.3 ± 2 | - |
| LSTRIST-CLO | 55.2 ± 10 | 87.0 ± 8 | 85 ± 7 |
| LSTRIST/AL10-CLO | 48.2 ± 7 | 88.2 ± 5 | 98 ± 2 |
| LSTRIST/AL1-CLO | 63.4 ± 9 | 97.8 ± 2 | 90 ± 8 |
| Formulation | MIC (ng/mL) ± s.d. a |
|---|---|
| LSTRIST-CLO | 23 ± 1.6 |
| LSTRIST/AL1-CLO | 17 ± 1.4 |
| LSTRIST | no activity |
| LSTRIST/AL1 | no activity |
| CLO | 32 ± 2.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160. https://doi.org/10.3390/polym10020160
Esposito E, Sguizzato M, Bories C, Nastruzzi C, Cortesi R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers. 2018; 10(2):160. https://doi.org/10.3390/polym10020160
Chicago/Turabian StyleEsposito, Elisabetta, Maddalena Sguizzato, Christian Bories, Claudio Nastruzzi, and Rita Cortesi. 2018. "Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment" Polymers 10, no. 2: 160. https://doi.org/10.3390/polym10020160
APA StyleEsposito, E., Sguizzato, M., Bories, C., Nastruzzi, C., & Cortesi, R. (2018). Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers, 10(2), 160. https://doi.org/10.3390/polym10020160