α-Cyclodextrins Polyrotaxane Loading Silver Sulfadiazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PR
2.3. Preparation of PR-(SD-Ag)
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. X-ray Diffraction
2.6. 1H NMR Spectroscopy
2.7. Light Stability
2.8. Analysis of Drug Content
2.9. In Vitro Release Studies
3. Results and Discussion
3.1. Preparation of the PR-(SD-Ag) Complex
3.2. Drug Loading and In Vitro Release
3.3. Antibacterial Studies and Light Stability
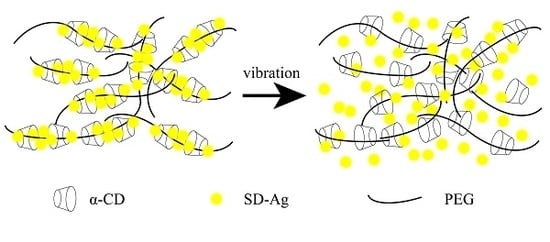
3.4. The Mechanism of PR Loading SD-Ag
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Heydari, B.; Khalili, H.; Karimzadeh, I.; Emadi-Kochak, H. Clinical, paraclinical, and antimicrobial resistance features of community-acquired acute bacterial meningitis at a large infectious diseases ward in Tehran, Iran. Iran. J. Pharm. Res. 2016, 15, 347–354. [Google Scholar] [PubMed]
- Niu, F.G.; Pan, W.C.; Su, Y.J.; Yang, Y.J. Physical and antimicrobial properties of thyme oil emulsions stabilized by ovalbumin and gum Arabic. Food Chem. 2016, 212, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.; Firdaous, L.; Chataigne, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M. Fever in critical neurologic illness. JAMA-J. Am. Med. Assoc. 2014, 312, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Stretton, S.; Gopinathan, U.; Willcox, M.D.P. Corneal ulceration in pediatric patients. Paediatr. Drugs 2002, 4, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2013, 114, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Fang, W.Y. Antimicrobial plastics. Fine Petrochem. 1998, 6, 14–18. [Google Scholar]
- Rai, M.; Yadav, A.; Gad, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.F.; Zhao, J.J.; Huang, J.; Xu, Y.M. Polyelectrolyte sponges with antimicrobial functions based on chitosan and sodium alginate. J. Wuhan Univ. Technol. 2006, 28, 25–28. [Google Scholar]
- Rosenkranz, H.S.; Carr, H.S. Silver sulfadiazine: Effect on the growth and metabolism of bacteria. Antimicrob. Agents Chemother. 1972, 2, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Kataoka, T.; Ito, K. Preparation of a “sliding graft copolymer”, an organic solvent-soluble polyrotaxane containing mobile side chains, and its application for a crosslinked elastomeric supramolecular film. Soft Matter 2008, 4, 245–249. [Google Scholar] [CrossRef]
- Araki, J.; Ito, K. Recent advances in the preparation of cyclodextrin-based polyrotaxanes and their applications to soft materials. Soft Matter 2007, 3, 1456–1473. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, M.; Liu, C.; Wei, C.; Wang, Y.; Sun, H.; Ye, H. A supramolecular strategy for self-mobile adsorption sites in affinity membrane. Macromol. Rapid Commun. 2014, 35, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Dam, H.H.; Kamphuis, M.M.; Richardson, J.J.; Caruso, F. Self-assembled stimuli-responsive polyrotaxane core-shell particles. Biomacromolecules 2014, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Nam, E.; Cui, J.; Xu, C.; Fu, Q.; Ren, J.M.; Wong, E.H.H.; Ladewig, K.; Caruso, F.; Blencowe, A.; et al. Fabrication of ultra-thin polyrotaxane-based films via solid-state continuous assembly of polymers. Chem. Commun. 2015, 51, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Kwon, Y.M.; Lee, W.K.; Park, Y.J.; Yang, V.C. In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide. J. Control. Release 2007, 124, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ye, L.; Ishihara, K.; Yui, N. Preparation and surface properties of polyrotaxane-containing tri-block copolymers as a design for dynamic biomaterials surfaces. Colloids Surf. B 2012, 89, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ooya, T.; Choi, H.S.; Yamashita, A.; Yui, N.; Sugaya, Y.; Kano, A.; Maruyama, A.; Akita, H.; Ito, R.; Kogure, K.; et al. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 2006, 128, 3852–3853. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Yui, N.; Ooya, T.; Kano, A.; Maruyama, A.; Akita, H.; Kogure, K.; Harashima, H. Synthesis of a biocleavable polyrotaxane-plasmid DNA (pDNA) polyplex and its use for the rapid nonviral delivery of pDNA to cell nuclei. Nat. Protoc. 2006, 1, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, T.; He, B.; Gu, Z. Self-assembly polyrotaxanes nanoparticles as carriers for anticancer drug methotrexate delivery. Nano-Micro Lett. 2014, 6, 108–115. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Preparation and properties of inclusion complexes of Poly(ethy1eneglycol) with α-cyclodextrin. Macromolecules 1993, 26, 5698–5703. [Google Scholar] [CrossRef]
- Rajendiran, N.; Venkatesh, G.; Saravanan, J. Supramolecular aggregates formed by sulfadiazine and sulfisomidine inclusion complexes with α- and β-cyclodextrins. Spectrochim. Acta A 2014, 129, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, N.; Thulasidhasan, J. Interaction of sulfanilamide and sulfamethoxazole with bovine serum albumin and adenine: Spectroscopic and molecular docking investigations. Spectrochim. Acta A 2015, 144, 183–191. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhong, C.; Wang, W.; Jia, Y.; Wang, L.; Ren, L. α-Cyclodextrins Polyrotaxane Loading Silver Sulfadiazine. Polymers 2018, 10, 190. https://doi.org/10.3390/polym10020190
Liu S, Zhong C, Wang W, Jia Y, Wang L, Ren L. α-Cyclodextrins Polyrotaxane Loading Silver Sulfadiazine. Polymers. 2018; 10(2):190. https://doi.org/10.3390/polym10020190
Chicago/Turabian StyleLiu, Sa, Chunting Zhong, Weiwei Wang, Yongguang Jia, Lin Wang, and Li Ren. 2018. "α-Cyclodextrins Polyrotaxane Loading Silver Sulfadiazine" Polymers 10, no. 2: 190. https://doi.org/10.3390/polym10020190
APA StyleLiu, S., Zhong, C., Wang, W., Jia, Y., Wang, L., & Ren, L. (2018). α-Cyclodextrins Polyrotaxane Loading Silver Sulfadiazine. Polymers, 10(2), 190. https://doi.org/10.3390/polym10020190