Affinity Capillary Electrochromatography of Molecularly Imprinted Thin Layers Grafted onto Silica Capillaries Using a Surface-Bound Azo-Initiator and Living Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
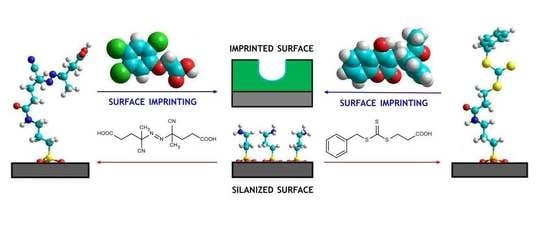
2.2. Silica Surface Functionalization
2.3. Silica Surface Derivatization with Azo-Initiator and RAFT Agent
2.4. Synthesis of MIP Thin Layers
2.5. Capillary Electrochromatography
3. Results and Discussion
3.1. Approaches to Surface Grafting
3.2. Study of Polymer-Ligand Recognition Properties
3.2.1. 2,4,5-T-Imprinted Capillary
3.2.2. Warfarin-Imprinted Capillary
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R. Bioapplications for molecularly imprinted polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 2016, 6, 88807–88819. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications, Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Heiden, P.A. Recent developments in molecularly imprinted nanoparticles by surface imprinting techniques. Macromol. Mater. Eng. 2014, 299, 268–282. [Google Scholar] [CrossRef]
- Kubo, T.; Otsuka, K. Recent progress in molecularly imprinted media by new preparation concepts and methodological approaches for selective separation of targeting compounds. Trends Anal. Chem. 2016, 81, 102–109. [Google Scholar] [CrossRef]
- Dyer, D.J. Photoinitiated synthesis of grafted polymers. Adv. Polym. Sci. 2006, 197, 47–65. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. Adv. Polym. Sci. 2006, 197, 1–45. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Sopper, O.; Haupt, K. Photopolymerization and photostructuring of molecularly imprinted polymers for sensor applications: A review. Anal. Chim. Acta 2012, 717, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Salian, V.D.; Byrne, M.E. Living radical polymerization and molecular imprinting: Improving polymer morphology in imprinted polymers. Macromol. Mater. Eng. 2013, 298, 379–390. [Google Scholar] [CrossRef]
- Iacob, B.C.; Bodoki, E.; Oprean, R. Recent advances in capillary electrochromatography using molecularly imprinted polymers. Electrophoresis 2014, 35, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.N.; Wei, Z.H.; Liu, Z.S. Current trends in the development of molecularly imprinted polymers in CEC. Electrophoresis 2015, 36, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A.; Han, K.M.; Hwang, D.G.; Cheong, W.J. Preparation of open tubular molecule imprinted polymer capillary columns with various templates by a generalized procedure and their chiral and non-chiral separation performance in CEC. Electrophoresis 2010, 31, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, C.; Anfossi, L.; Giovannoli, C.; Tozzi, C. Binding properties of 2,4,5-trichlorophenoxyacetic acid-imprinted polymers prepared with different molar ratios between template and functional monomer. Talanta 2004, 62, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.C.G.; Rosengren, A.M.; Naslund, I.; Andersson, P.O.; Nicholls, I.A. Synthetic human serum albumin Sudlow I binding site mimics. J. Med. Chem. 2010, 53, 7932–7937. [Google Scholar] [CrossRef] [PubMed]
- Benagli, M.; Alberti, A.; Spisni, E.; Papi, A.; Treossi, E.; Palermo, V. Polymeric micelles using pseudo-amphiphilic block copolymers and their cellular uptake. J. Mater. Chem. 2011, 21, 2555–2562. [Google Scholar] [CrossRef]
- Adbo, K.; Nicholls, I.A. Enantioselective solid-phase extraction using Tröger’s base molecularly imprinted polymers. Anal. Chim. Acta 2001, 435, 115–120. [Google Scholar] [CrossRef]
- Schweitz, L. Molecularly imprinted polymer coatings for open-tubular capillary electrochromatography prepared by surface initiation. Anal. Chem. 2002, 74, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Mu, L.; Pang, Q.; Huang, Y.; Liu, Z. Preparation and characterization of grafted imprinted monolith for capillary electrochromatography. Electrophoresis 2012, 33, 3021–3027. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannoli, C.; Passini, C.; Nardo, F.D.; Anfossi, L.; Baggiani, C.; Nicholls, I.A. Affinity Capillary Electrochromatography of Molecularly Imprinted Thin Layers Grafted onto Silica Capillaries Using a Surface-Bound Azo-Initiator and Living Polymerization. Polymers 2018, 10, 192. https://doi.org/10.3390/polym10020192
Giovannoli C, Passini C, Nardo FD, Anfossi L, Baggiani C, Nicholls IA. Affinity Capillary Electrochromatography of Molecularly Imprinted Thin Layers Grafted onto Silica Capillaries Using a Surface-Bound Azo-Initiator and Living Polymerization. Polymers. 2018; 10(2):192. https://doi.org/10.3390/polym10020192
Chicago/Turabian StyleGiovannoli, Cristina, Cinzia Passini, Fabio Di Nardo, Laura Anfossi, Claudio Baggiani, and Ian A. Nicholls. 2018. "Affinity Capillary Electrochromatography of Molecularly Imprinted Thin Layers Grafted onto Silica Capillaries Using a Surface-Bound Azo-Initiator and Living Polymerization" Polymers 10, no. 2: 192. https://doi.org/10.3390/polym10020192
APA StyleGiovannoli, C., Passini, C., Nardo, F. D., Anfossi, L., Baggiani, C., & Nicholls, I. A. (2018). Affinity Capillary Electrochromatography of Molecularly Imprinted Thin Layers Grafted onto Silica Capillaries Using a Surface-Bound Azo-Initiator and Living Polymerization. Polymers, 10(2), 192. https://doi.org/10.3390/polym10020192