Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries
Abstract
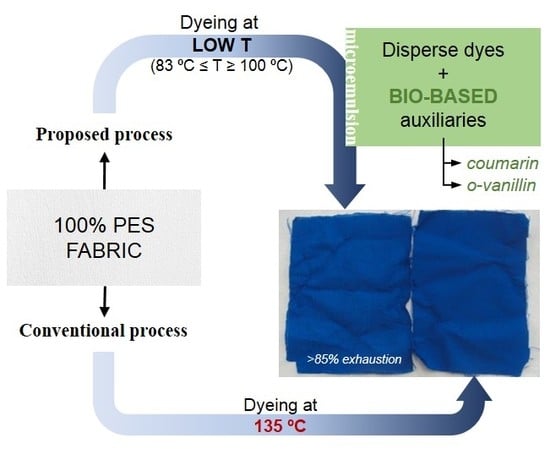
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Methods
2.2.1. Dyeing Procedure
2.2.2. Colour Fastness
2.2.3. Dye Absorption
2.2.4. Particle Size Analysis
2.2.5. Determination of Exhaustion
2.2.6. Determination of Colour Strength
3. Results and Discussion
3.1. Emulsion Particle Size
3.2. Dyeing Kinetics and Rate Constant
3.3. Activation Energy
3.4. Washing Fastness
3.5. Hot Pressing Fastness
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gacén, J. Fibras Químicas, 2nd ed.; Universitat Politècnica de Catalunya: Barcelona, Spain, 1991. [Google Scholar]
- Datyner, A. The Solubilization of Disperse Dyes by Dispersing Agents at 127 °C. J. Soc. Dyers Colour. 1978, 94, 256–260. [Google Scholar] [CrossRef]
- Weingarten, R. Theoretische Betrachtungen über das Fárben von Polyesterfasern mit Dispersionsfarbstoffen und ihre Bedeutung für die Praxis. Text. Prax. Inst. 1979, 28, 340–397. [Google Scholar]
- Saligram, A.N.; Shukla, S.R.; Mathur, M. Dyeing of Polyester Fibers Using Ultrasound. J. Soc. Dyers Colour. 1993, 109, 263–266. [Google Scholar] [CrossRef]
- Ingamells, W.; Yabani, A. The Swelling and Plasticization of Poly(ethylene terephthalate) during Carrier Dyeing. J. Soc. Dyers Colour. 1977, 93, 417–423. [Google Scholar] [CrossRef]
- Peters, R.H. Textile Chemistry 2; Elsevier: Amsterdam, The Netherlands, 1975; Volume III. [Google Scholar]
- Harifi, T.; Montazer, M. Free carrier dyeing of polyester fabric using nano TiO2. Dyes Pigment. 2013, 97, 440–445. [Google Scholar] [CrossRef]
- Choi, T.S.; Shimizu, Y.; Shirai, H.; Hamada, K. Disperse dyeing of polyester fiber using gemini surfactants containing ammonium cations as auxiliaries. Dyes Pigment. 2001, 50, 55–65. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibres with disperse dyes in supercritical CO2. Dyes Pigment. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- Pasquet, V.; Perwuelz, A.; Behary, N.; Isaad, J. Vanillin, a potential carrier for low temperature dyeing of polyester fabrics. J. Clean. Prod. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Fité, F.J.C. Dyeing Polyester at Low Temperature: Kinetics of Dyeing with Disperse Dyes. Text. Res. J. 1995, 65, 362–367. [Google Scholar] [CrossRef]
- Kim, J.P. The Effect of Carriers on the Reduction of Glass Transition Temperature and Dyeing of an Arylic Fiber. Hang’guk Somyu Konghakholchi 1994, 31, 608–612. [Google Scholar]
- Ahmad, W.Y.W.; Lomas, M. Low Temperature Dyeing of Polyester Fibers using Ultrasound. J. Soc. Dyers Colour. 1996, 112, 245–248. [Google Scholar] [CrossRef]
- Carrion, F.J. Dyeing polyester at low temperature with disperse dyes and ecological auxiliaries. In Proceedings of the XXIV International Congress of the IFATCC (International Federation of Associations of Textile Chemists and Colourists), Prague, Czech Republic, 13–16 June 2016. [Google Scholar]
- Carrion, F.J.; Radei, S. Development auxiliaries for dyeing polyester at low temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 082006. [Google Scholar] [CrossRef]
- Cha, T.S.; Chen, C.F.; Yee, W.; Aziz, A.; Loh, S.H. Cinnamic acid, coumarin and vanillin: Alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J. Microbiol. Methods 2011, 84, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposome Technology, Vol. I, Preparation of Liposomes; CRC Press Inc.: Boca Raton, FL, USA, 1984. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Otutu, J.O.; Ameuru, U.S. Thermodynamic Sorption Parameters and Kinetics of Dyeing Disazo Dyes Derived from 4-aminobenzoic acid and 4-amino-3-nitrotoluene on Polyester Fibre and Polyamide Fibre. Br. J. Appl. Sci. Technol. 2014, 4, 2955–2969. [Google Scholar] [CrossRef]
- Textiles—Test for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering; ISO 105-C06:2010; adapted to Spanish by UNE-EN ISO 105-C06; International Organization for Standardization: Geneva, Switzerland, 2010.





| Dyes and Chemicals | Chemical Structure |
|---|---|
| C.I. Disperse Red 167 CAS No. = 61968-52-3/26850-12-4 MW = 519.93 g·mol–1 |  |
| C.I. Disperse blue 79 CAS No. = 12239-34-8 MW = 639.41 g·mol−1 |  |
| Coumarin 2H-chromen-2-one CAS No. = 91-64-5 MW = 146.15 g·mol−1 |  |
| o-vanillin 2-hydroxy-3-methoxybenzaldehyde CAS No. = 148-53-8 MW = 152.15 g·mol−1 |  |
| Dye-Bath | Average Diameter (nm) | Polydispersity |
|---|---|---|
| 2% o.w.f. Disperse Red 167; 4% o.w.f. coumarin & 1 mL/g o.w.f. n-buthylacetate | 183 ± 9.5 | 153 ± 0.03 |
| 2% o.w.f. Disperse Blue 79; 4% o.w.f. coumarin & 1 mL/g o.w.f. n-buthylacetate | 160.3 ± 14.2 | 0.121 ± 0.03 |
| 2% o.w.f. Disperse Red 167; 4% o.w.f. o-vanillin & 1 mL/g o.w.f. n-buthylacetate | 236.3 ± 10.97 | 0.107 ± 0.032 |
| 2% o.w.f. Disperse Blue 79; 4% o.w.f. o-vanillin & 1 mL/g o.w.f. n-buthylacetate | 249.3 ± 4.5 | 0.247 ± 0.05 |
| 2% o.w.f. Disperse Red 167 & 1 mL/g o.w.f. n-buthylacetate | 238.3 ± 0.6 | 0.183 ± 0.01 |
| 2% o.w.f. Disperse Blue 79 & 1 mL/g o.w.f. n-buthylacetate | 250 ± 15.5 | 0.3 ± 0.05 |
| T = 100 °C C.I. Disperse Red 167 | |||||||
|---|---|---|---|---|---|---|---|
| t (min) | 5 | 10 | 30 | 50 | 70 | 90 | 105 |
| Coumarin | |||||||
| K/S λ = 640 nm | 4.490 | 8.820 | 11.157 | 16.382 | 17.025 | 18.698 | 19.133 |
| Vanillin | |||||||
| K/S λ = 640 nm | 3.487 | 9.260 | 13.190 | 14.934 | 18.378 | 19.305 | 21.383 |
| T = 100 °C C.I. Disperse Blue 79 | |||||||
|---|---|---|---|---|---|---|---|
| t (min) | 5 | 10 | 30 | 50 | 70 | 90 | 105 |
| Coumarin | |||||||
| K/S λ = 640 nm | 3.298 | 4.807 | 3.298 | 4.807 | 3.298 | 4.807 | 3.298 |
| Vanillin | |||||||
| K/S λ = 640 nm | 3.877 | 5.061 | 6.982 | 7.741 | 9.982 | 11.166 | 12.08 |
| o-Vanillin | Coumarin | |||||
|---|---|---|---|---|---|---|
| Disperse Dye | K | R2 | Exhaustion after 120 min (%) | K | R2 | Exhaustion after 120 min (%) |
| C.I. Disperse Red 167 at 100 °C | 0.5667 | 0.9855 | 88.21 | 0.1759 | 0.9659 | 81.22 |
| C.I. Disperse Red 167 at 95 °C | 0.1712 | 0.9716 | 79.78 | 0.0711 | 0.9704 | 71.45 |
| C.I. Disperse Red 167 at 90 °C | 0.0426 | 0.992 | 65.65 | 0.0312 | 0.989 | 61.96 |
| C.I. Disperse Red 167 at 83 °C | 0.0128 | 0.9523 | 50.29 | 0.0153 | 0.9436 | 53.45 |
| C.I. Disperse Blue 79 at 100 °C | 0.472 | 0.9764 | 87.96 | 0.1892 | 0.9577 | 81.42 |
| C.I. Disperse Blue 79 at 95 °C | 0.1359 | 0.9736 | 78.42 | 0.0638 | 0.9268 | 71.37 |
| C.I. Disperse Blue 79 at 90 °C | 0.0722 | 0.9305 | 71.95 | 0.0294 | 0.934 | 63.39 |
| C.I. Disperse Blue 79 at 83 °C | 0.0208 | 0.9108 | 58.34 | 0.0129 | 0.9105 | 53.60413 |
| o-Vanillin | Coumarin | |||
|---|---|---|---|---|
| Disperse Dyes | E (Kcal·mol–1) | R2 | E (Kcal·mol–1) | R2 |
| C.I. Disperse Red 167 | 59.3 | 0.9869 | 37.8 | 0.9763 |
| C.I. Disperse Blue 79 | 46.6 | 0.9841 | 40.8 | 0.9781 |
| PET Fabric Dyed 120 min | o-Vanillin | Coumarin | ||||
|---|---|---|---|---|---|---|
| Gray Scale | PES | Cotton | Gray Scale | PES | Cotton | |
| C.I. Disperse Red 167 at 83 °C | 4–5 | 4 | 3–4 | 5 | 4 | 3–4 |
| C.I. Disperse Red 167 at 90 °C | 5 | 3–4 | 3–4 | 4–5 | 4–5 | 3–4 |
| C.I. Disperse Red 167 at 95 °C | 5 | 4–5 | 3–4 | 4–5 | 4 | 3–4 |
| C.I. Disperse Red 167 at 100 °C | 5 | 4–5 | 3–4 | 5 | 4 | 3–4 |
| C.I. Disperse Blue 79 at 83 °C | 4–5 | 4–5 | 3–4 | 4–5 | 4–5 | 3–4 |
| C.I. Disperse Blue 79 at 90 °C | 4–5 | 4–5 | 3–4 | 4–5 | 4–5 | 3–4 |
| C.I. Disperse Blue 79 at 95 °C | 5 | 5 | 3–4 | 5 | 5 | 3–4 |
| C.I. Disperse Blue 79 at 100 °C | 5 | 4–5 | 3 | 5 | 4–5 | 3–4 |
| PET Fabric Dyed 120 min | Discharge Hot Pressing Fastness (Gray Scale) | |||||
|---|---|---|---|---|---|---|
| o-Vanillin | Coumarin | |||||
| 110 °C | 150 °C | 200 °C | 110 °C | 150 °C | 200 °C | |
| C.I. Disperse Red 167 at 83 °C | 5 | 5 | 5 | 5 | 5 | 4–5 |
| C.I. Disperse Red 167 at 90 °C | 5 | 5 | 4–5 | 5 | 5 | 4–5 |
| C.I. Disperse Red 167 at 95 °C | 5 | 5 | 4–5 | 5 | 4–5 | 4–5 |
| C.I. Disperse Red 167 at 100 °C | 5 | 4–5 | 4–5 | 5 | 4–5 | 4 |
| C.I. Disperse Blue 79 at 83 °C | 5 | 4–5 | 4–5 | 5 | 5 | 4–5 |
| C.I. Disperse Blue 79 at 90 °C | 5 | 5 | 4–5 | 5 | 5 | 4–5 |
| C.I. Disperse Blue 79 at 95 °C | 5 | 4–5 | 4–5 | 5 | 5 | 4–5 |
| C.I. Disperse Blue 79 at 100 °C | 5 | 5 | 5 | 5 | 5 | 5 |
| PET Fabric Dyed 120 min | Degradation Hot Pressing Fastness (Gray Scale) | |||||
|---|---|---|---|---|---|---|
| o-Vanillin | Coumarin | |||||
| 110 °C | 150 °C | 200 °C | 110 °C | 150 °C | 200 °C | |
| C.I. Disperse Red 167 at 83 °C | 4–5 | 3 | 2 | 3 | 2–3 | 1–2 |
| C.I. Disperse Red 167 at 90 °C | 4–5 | 3 | 1–2 | 3 | 2–3 | 1–2 |
| C.I. Disperse Red 167 at 95 °C | 4–5 | 4 | 2 | 2–3 | 2–3 | 2–3 |
| C.I. Disperse Red 167 at 100 °C | 4–5 | 4 | 2 | 4 | 4 | 4–5 |
| C.I. Disperse Blue 79 at 83 °C | 5 | 3 | 1–2 | 3–4 | 3 | 1–2 |
| C.I. Disperse Blue 79 at 90 °C | 4 | 3–4 | 1–2 | 3–4 | 2–3 | 2 |
| C.I. Disperse Blue 79 at 90 °C | 4–5 | 4–5 | 2–3 | 3 | 2 | 3 |
| C.I. Disperse Blue 79 at 100 °C | 4–5 | 4–5 | 2–3 | 4–5 | 4–5 | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radei, S.; Carrión-Fité, F.J.; Ardanuy, M.; Canal, J.M. Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries. Polymers 2018, 10, 200. https://doi.org/10.3390/polym10020200
Radei S, Carrión-Fité FJ, Ardanuy M, Canal JM. Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries. Polymers. 2018; 10(2):200. https://doi.org/10.3390/polym10020200
Chicago/Turabian StyleRadei, Shahram, F. Javier Carrión-Fité, Mònica Ardanuy, and José María Canal. 2018. "Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries" Polymers 10, no. 2: 200. https://doi.org/10.3390/polym10020200
APA StyleRadei, S., Carrión-Fité, F. J., Ardanuy, M., & Canal, J. M. (2018). Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries. Polymers, 10(2), 200. https://doi.org/10.3390/polym10020200