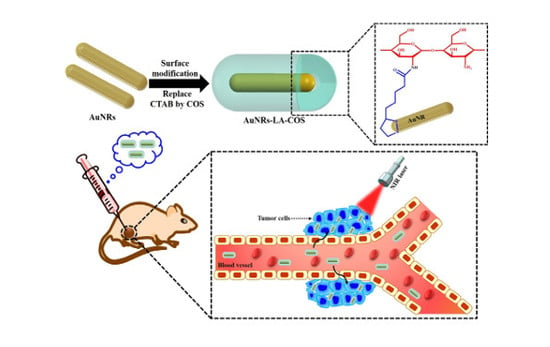
Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Surface Modification of Gold Nanorods
2.2. Stability Studies
2.3. In Vitro Photothermal Heating Characterization
2.4. Cell Viability Assay
2.5. In Vitro Photothermal Ablation of MDA-MB-231 Cells
2.6. In Vivo Photothermal Ablation of MDA-MB-231 Cells
2.7. Histology Analysis
3. Results and Discussion
3.1. Surface Modification of AuNRs
3.2. Characterization
3.3. Stability Studies
3.4. Photothermal Conversion of AuNRs-LA-COS
3.5. Biocompatibility Study
3.6. In Vitro Cytotoxicity Study
3.7. In Vitro Photothermal Ablation of MDA-MB-231 Cells
3.8. In Vivo Photothermal Ablation of MDA-MB-231 Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jang, B.; Park, S.; Kang, S.H.; Kim, J.K.; Kim, S.-K.; Kim, I.-H.; Choi, Y. Gold nanorods for target selective spect/ct imaging and photothermal therapy in vivo. Quant. Imaging Med. Surg. 2012, 2, 1–15. [Google Scholar] [PubMed]
- Phillips, D. Light relief: Photochemistry and medicine. Photochem. Photobiol. Sci. 2010, 9, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.-Y.; Zhang, H.; Xia, Y.; Li, X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, X.; Wu, M.; Lin, R.; Li, B.; Liu, J. SPION@Cu2−xS nanoclusters for highly sensitive mri and targeted photothermal therapy of hepatocellular carcinoma. J. Mater. Chem. B 2016, 4, 4119–4129. [Google Scholar] [CrossRef]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, X.; Li, H.; Zhao, Z.; Chi, X.; Huang, G.; Huang, D.; Liu, G.; Wang, X.; Gao, J. Theranostic au cubic nano-aggregates as potential photoacoustic contrast and photothermal therapeutic agents. Theranostics 2014, 4, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting mri and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Rong, P.; Yang, K.; Huang, P.; Sun, K.; Chen, X. Semimetal nanomaterials of antimony as highly efficient agent for photoacoustic imaging and photothermal therapy. Biomaterials 2015, 45, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.P.; Chen, H.; Wang, J.; Ong, H.C.; Leung, K.C.-F.; Ho, H.P.; Kong, S.K. In vitro effect of ctab-and peg-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology 2012, 6, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Niidome, Y.; Niidome, T.; Kaneko, K.; Kawasaki, H.; Yamada, S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir 2006, 22, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Shatanawi, A.; Kurtz, T.; Caldwell, R.B.; Caldwell, R.W. Toxicity and cellular uptake of gold nanorods in vascular endothelium and smooth muscles of isolated rat blood vessel: Importance of surface modification. Small 2012, 8, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Leonov, A.P.; Zheng, J.; Clogston, J.D.; Stern, S.T.; Patri, A.K.; Wei, A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano 2008, 2, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Parab, H.J.; Chen, H.M.; Lai, T.-C.; Huang, J.H.; Chen, P.H.; Liu, R.-S.; Hsiao, M.; Chen, C.-H.; Tsai, D.-P.; Hwu, Y.-K. Biosensing, cytotoxicity, and cellular uptake studies of surface-modified gold nanorods. J. Phys. Chem. C 2009, 113, 7574–7578. [Google Scholar] [CrossRef]
- Charan, S.; Sanjiv, K.; Singh, N.; Chien, F.-C.; Chen, Y.-F.; Nergui, N.N.; Huang, S.-H.; Kuo, C.W.; Lee, T.-C.; Chen, P. Development of chitosan oligosaccharide-modified gold nanorods for in vivo targeted delivery and noninvasive imaging by nir irradiation. Bioconjugate Chem. 2012, 23, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.-O.; Seo, H.; Oh, J. Marine biopolymer-based nanomaterials as a novel platform for theranostic applications. Polym. Rev. 2017, 4, 631–667. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially n-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Richardson, S.W.; Kolbe, H.J.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Controll. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.H.; Susumu, K.; Mei, B.C.; Medintz, I.L.; Delehanty, J.B.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Multidentate poly (ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J. Am. Chem. Soc. 2010, 132, 9804–9813. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J. Am. Chem. Soc. 2005, 127, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded high yield synthesis of short au nanorods in aqueous solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Z.; Xu, L.; Xu, R.; Lu, X. Facile purification of colloidal nir-responsive gold nanorods using ions assisted self-assembly. Nanoscale Res. Lett. 2011, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Oh, Y.-O.; Song, K.; Seo, H.; Oh, J. Anti-egfr antibody conjugation of fucoidan-coated gold nanorods as novel photothermal ablation agents for cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 14633–14646. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, H.; Liu, G.; Zhou, W.; Chen, Y.; Jin, Q.; Ji, J. Multidentate zwitterionic chitosan oligosaccharide modified gold nanoparticles: Stability, biocompatibility and cell interactions. Nanoscale 2013, 5, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-Q.; Zhao, M.-D.; Yuan, H.; You, J.; Du, Y.-Z.; Zeng, S. A novel chitosan oligosaccharide–stearic acid micelles for gene delivery: Properties and in vitro transfection studies. Int. J. Pharm. 2006, 315, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ji, Y.; Meng, J.; Wu, X.; Xu, H. Probing the behaviors of gold nanorods in metastatic breast cancer cells based on uv-vis-nir absorption spectroscopy. PLoS ONE 2012, 7, e31957. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, S.; Yang, S.-M.; Park, H.G. Detection of DNA immobilization and hybridization on gold/silver nanostructures using localized surface plasmon resonance. J. Nanosci. Nanotechnol. 2009, 9, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Emam, A.; Mohamed, M.; Girgis, E.; Rao, K.V. Hybrid magnetic-plasmonic nanocomposite: Embedding cobalt clusters in gold nanorods. RSC Adv. 2015, 5, 34696–34703. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Yang, C.-F.; Feng, S.-W. Electrochemical formation of crooked gold nanorods and gold networked structures by the additive organic solvent. J. Colloid Interface Sci. 2007, 306, 56–65. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Mondal, S.; Nguyen, T.P.; Kim, H.; Phan, T.T.V.; Lee, K.D.; Oh, J. Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells. Polymers 2018, 10, 232. https://doi.org/10.3390/polym10030232
Manivasagan P, Bharathiraja S, Santha Moorthy M, Mondal S, Nguyen TP, Kim H, Phan TTV, Lee KD, Oh J. Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells. Polymers. 2018; 10(3):232. https://doi.org/10.3390/polym10030232
Chicago/Turabian StyleManivasagan, Panchanathan, Subramaniyan Bharathiraja, Madhappan Santha Moorthy, Sudip Mondal, Thanh Phuoc Nguyen, Hyehyun Kim, Thi Tuong Vy Phan, Kang Dae Lee, and Junghwan Oh. 2018. "Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells" Polymers 10, no. 3: 232. https://doi.org/10.3390/polym10030232
APA StyleManivasagan, P., Bharathiraja, S., Santha Moorthy, M., Mondal, S., Nguyen, T. P., Kim, H., Phan, T. T. V., Lee, K. D., & Oh, J. (2018). Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells. Polymers, 10(3), 232. https://doi.org/10.3390/polym10030232