Influence of Selectively Localised Nanoclay Particles on Non-Isothermal Crystallisation and Degradation Behaviour of PP/LDPE Blend Composites
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Sample Preparation
2.3. Characterisation Techniques
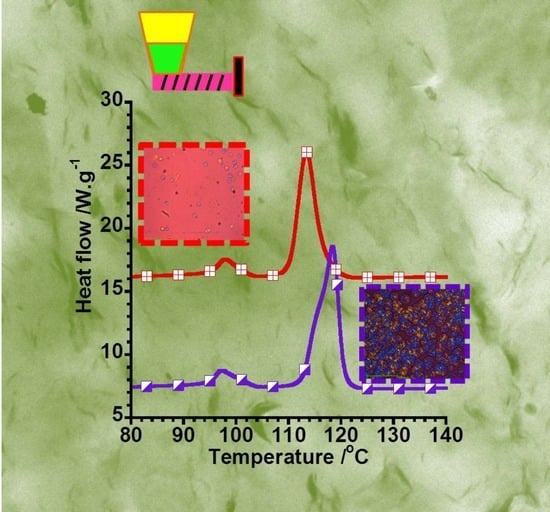
3. Results and Discussion
3.1. Melting and Cooling Properties
3.2. Non-Isothermal Crystallisation Kinetics
3.3. Calculation of the Activation Energy for Non-Isothermal Crystal Growth
3.4. Polarised Optical Microscopy
3.5. Thermal Degradation Kinetics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jose, S.; Aprem, A.S.; Francis, B.; Chandy, M.C.; Werner, P.; Alstaedt, V.; Thomas, S. Phase morphology, crystallization behavior and mechanical properties of isotactic polypropylene/high density polyethylene blends. Eur. Polym. J. 2004, 40, 2105–2115. [Google Scholar] [CrossRef]
- Li, J.; Shanks, R.A.; Long, Y. Mechanical properties and morphology of polyethylene-polypropylene blends with controlled thermal history. J. Appl. Polym. Sci. 2000, 76, 1151–1164. [Google Scholar] [CrossRef]
- Tai, C.M.; Li, R.K.Y.; Ng, C.N. Impact behaviour of polypropylene/polyethylene blends. Polym. Test. 2000, 19, 143–154. [Google Scholar] [CrossRef]
- Zhou, M.; Mi, D.; Hou, F.; Zhang, J. Tailored Crystalline Structure and Mechanical Properties of Isotactic Polypropylene/High Molecular Weight Polyethylene Blend. Ind. Eng. Chem. Res. 2017, 56, 8385–8392. [Google Scholar] [CrossRef]
- Zhang, X.M.; Ajji, A. Oriented structure of PP/LLDPE multilayer and blends films. Polymer 2005, 46, 3385–3393. [Google Scholar] [CrossRef]
- Mofokeng, T.G.; Ojijo, V.; Ray, S.S. The Influence of Blend Ratio on the Morphology, Mechanical, Thermal and Rheological Properties of PP/LDPE Blends. Macromol. Mater. Eng. 2016, 301, 1191–1201. [Google Scholar] [CrossRef]
- Márquez, Y.; Franco, L.; Turon, P.; Martínez, J.C.; Puiggalí, J. Study of Non-Isothermal Crystallization of Polydioxanone and Analysis of Morphological Changes Occurring during Heating and Cooling Processes. Polymers 2016, 8, 351. [Google Scholar] [CrossRef]
- Xu, W.; Liang, G.; Zhai, H.; Tang, S.; Hang, G.; Pan, W.P. Preparation and crystallization behaviour of PP/PP-g-MAH/Org-MMT nanocomposite. Eur. Polym. J. 2003, 39, 1467–1474. [Google Scholar] [CrossRef]
- Yuan, Q.; Awate, S.; Misra, R.D.K. Nonisothermal crystallization behavior of polypropylene–clay nanocomposites. Eur. Polym. J. 2006, 42, 1994–2003. [Google Scholar] [CrossRef]
- Nagendra, B.; Mohan, K.; Gowd, E.B. Polypropylene/Layered double hydroxide (LDH) Nanocomposites: Influence of LDH particle size on the crystallization behavior of polypropylene. ACS Appl. Mater. Interfaces 2015, 7, 12399–12410. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.M.; Chen, W.C.; Zhu, X.S. Melt mixed compatibilized polypropylene/clay nanocomposites: Part 1—The effect of compatibilizers on optical transmittance and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2009, 40, 754–765. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Zhao, X.; Horiuchi, S.; Li, Y. Immiscible polymer blends compatibilized with reactive hybrid nanoparticles: Morphologies and properties. Polymer 2017, 132, 353–361. [Google Scholar] [CrossRef]
- Ojijo, V.; Cele, H.; Ray, S.S. Morphology and properties of polymer composites based on biodegradable polylactide/poly[(butylene succinate)-coadipate] blend and nanoclay. Macromol. Mater. Eng. 2011, 296, 865–877. [Google Scholar] [CrossRef]
- Hasegawa, N.; Kawasumi, M.; Kato, M.; Usuki, A.; Okada, A. Preparation and mechanical properties of polypropylene-clay hybrids using a maleic anhydride-modified polypropylene oligomer. J. Appl. Polym. Sci. 1998, 67, 87–92. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Ray, S.S. Effect of nanoclay on the nonisothermal crystallization of poly(propylene) and its blend with poly[(butylene succinate)-co-adipate]. Mol. Cryst. Liq. Cryst. 2012, 556, 176–190. [Google Scholar] [CrossRef]
- Goodarzi, V.; Jafari, S.H.; Khonakdar, H.A.; Monemian, S.A.; Mortazavi, M. An assessment of the role of morphology in thermal/thermo-oxidative degradation mechanism of PP/EVA/clay nanocomposites. Polym. Degrad. Stab. 2010, 95, 859–869. [Google Scholar] [CrossRef]
- Goodarzi, V.; Jafari, S.H.; Khonakdar, H.A.; Monemian, S.A.; Hässler, R.; Jehnichen, D. Nonisothermal crystallization kinetics and determination of surface-folding free energy of PP/EVA/OMMT nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 674–684. [Google Scholar] [CrossRef]
- Mofokeng, T.G.; Ray, S.S.; Ojijo, V. Structure–property relationship in PP/LDPE blend composites: The role of nanoclay localization. J. Appl. Polym. Sci. 2018, 135, 46193. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. De Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Bioki, H.A.; Mirbagheri, Z.A.; Tabbakh, F.; Mirjalili, G. Effect of crystallinity and irradiation on thermal properties and specific heat capacity of LDPE & LDPE/EVA. Appl. Radiat. Isot. 2012, 70, 1–5. [Google Scholar]
- Na, B.; Wang, K.; Zhang, Q.; Du, R.; Fu, Q. Tensile properties in the oriented blends of high-density polyethylene and isotactic polypropylene obtained by dynamic packing injection molding. Polymer 2005, 46, 3190–3198. [Google Scholar] [CrossRef]
- Li, J.; Shanks, R.A.; Olley, R.H.; Greenway, G.R. Miscibility and isothermal crystallisation of polypropylene in polyethylene melts. Polymer 2001, 42, 7685–7694. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of nonisothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Nat. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Baghaei, B.; Jafari, S.H.; Khonakdar, H.A.; Rezaeian, I.; As’habi, L.; Ahmadian, S. Interfacially compatibilized LDPE/POE blends reinforced with nanoclay: Investigation of morphology, rheology and dynamic mechanical properties. Polym. Bull. 2009, 62, 255–270. [Google Scholar] [CrossRef]
- Goodarzi, V.; Jafari, S.H.; Khonakdar, H.A.; Seyfi, J. Morphology, rheology and dynamic mechanical properties of PP/EVA/clay nanocomposites. J. Polym. Res. 2011, 18, 1829–1839. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Gang, W. Study on nonisothermal crystallization of maleic anhydride grafted polypropylene/montmorillonite nanocomposite. Polym. Test. 2003, 22, 217–223. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Crystallization behavior of poly[(butylene succinate)-co-adipate] nanocomposite. Macromol. Chem. Phys. 2006, 207, 1207–1219. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Hato, M.J.; Ray, S.S.; Luyt, A.S. Nanocomposites based on polyethylene and polyhedral oligomeric silsesquioxanes, 1−Microstructure, thermal and thermomechanical properties. Macromol. Mater. Eng. 2008, 293, 752–762. [Google Scholar] [CrossRef]
- Ray, S.S. New possibility for microstructural investigation of clay-based polymer nanocomposite by focused ion beam tomography. Polymer 2010, 51, 3966–3970. [Google Scholar] [CrossRef]
- Palacios, J.; Perera, R.; Rosales, C.; Albano, C.; Pastor, J.M. Thermal degradation kinetics of PP/OMMT nanocomposites with mPE and EVA. Polym. Degrad. Stab. 2012, 97, 729–737. [Google Scholar] [CrossRef]





| PP/LDPE/PP-g-MA/PE-g-MA/Clay | PP/LDPE Ratio (wt %/wt %) | PP-g-MA (wt %) | PE-g-MA (wt %) | OMMT (wt %) |
|---|---|---|---|---|
| 100/0/0/0/0 | 100/0 | 0 | 0 | 0 |
| 96/0/0/0/4 | 96/0 | 0 | 0 | 4 |
| 80/20/0/0/0 | 80/20 | 0 | 0 | 0 |
| 80/20/0/0/4 | 80/20 | 0 | 0 | 4 |
| 80/20/5/0/4 | 80/20 | 5 | 0 | 4 |
| 80/20/0/5/4 | 80/20 | 0 | 5 | 4 |
| 80/20/5/5/4 | 80/20 | 5 | 5 | 4 |
| 0/96/0/0/4 | 0/96 | 0 | 0 | 4 |
| 0/100/0/0/0 | 0/100 | 0 | 0 | 0 |
| Sample | Tim (°C) | Tfm (°C) | Tic (°C) | Tfc (°C) | ΔHim (J·g−1) | ΔHfm (J·g−1) | ||
|---|---|---|---|---|---|---|---|---|
| 100/0/0/0/0 | − | 163.9 ± 0.3 | − | 119.1 ± 0.5 | − | 109.2 ± 1.1 | 52.8 ± 0.5 | − |
| 96/0/0/0/4 | − | 163.2 ± 0.2 | − | 120.3 ± 0.1 | − | 95.4 ± 3.3 | 48.0 ± 1.7 | − |
| 91/0/5/0/4 | − | 163.9 ± 0.1 | − | 118.4 ± 0.3 | − | 96.3 ± 2.0 | 48.5 ± 1.0 | − |
| 0/100/0/0/0 | 109.6 ± 0.1 | − | 97.6 ± 0.2 | − | 130.1 ± 0.7 | − | − | 45.2 ± 0.2 |
| 0/96/0/0/4 | 109.6 ± 0.2 | − | 97.5 ± 0.2 | − | 125.5 ± 5.3 | − | − | 45.4 ± 1.9 |
| 0/91/0/5/4 | 109.5 ± 0.2 | − | 97.8 ± 0.2 | − | 119.8 ± 1.6 | − | − | 43.3 ± 0.5 |
| 80/20/0/0/0 | 108.7 ± 0.3 | 163.4 ± 0.6 | 96.9 ± 0.5 | 118.4 ± 0.3 | 17.1 ± 0.9 | 75.7 ± 2.5 | 45.7 ± 1.5 | 29.7 ± 1.6 |
| 80/20/0/0/4 | 108.5 ± 0.1 | 163.5 ± 0.3 | 97.4 ± 0.1 | 118.5 ± 0.2 | 17.0 ± 0.7 | 79.1 ± 2.0 | 49.8 ± 1.3 | 30.7 ± 1.3 |
| 80/20/5/0/4 | 108.8 ± 0.1 | 163.5 ± 0.3 | 97.3 ± 0.1 | 116.9 ± 0.4 | 13.2 ± 0.6 | 70.1 ± 3.5 | 43.5 ± 2.1 | 25.2 ± 1.1 |
| 80/20/0/5/4 | 108.8 ± 0.1 | 162.9 ± 0.2 | 98.2 ± 0.2 | 113.6 ± 0.1 | 16.6 ± 0.7 | 65.0 ± 1.1 | 43.1 ± 0.7 | 24.9 ± 0.1 |
| 80/20/5/5/4 | 108.8 ± 0.1 | 162.9 ± 0.3 | 97.6 ± 0.3 | 114.6 ± 0.2 | 14.7 ± 1.1 | 64.5 ± 1.5 | 42.2 ± 1.0 | 23.0 ± 1.7 |
| PP/LDPE/PP-g-MA/PE-g-MA/Clay | Kinetic Parameter | Degree of Crystallinity | ||
|---|---|---|---|---|
| 25% | 40% | 60% | ||
| 100/0/0/0/0 | F(T) | 6.298 | 7.263 | 8.341 |
| α | 1.057 | 1.062 | 1.072 | |
| 96/0/0/0/4 | F(T) | 7.936 | 8.872 | 9.761 |
| α | 0.948 | 0.943 | 0.994 | |
| 91/0/5/0/4 | F(T) | 7.17 | 8.213 | 8.438 |
| α | 0.944 | 0.942 | 1.003 | |
| 80/20/0/0/0 | F(T) | 6.563 | 7.514 | 8.611 |
| α | 0.975 | 0.956 | 0.919 | |
| 80/20/0/0/4 | F(T) | 4.342 | 4.945 | 5.995 |
| α | 1.186 | 1.223 | 1.192 | |
| 80/20/5/0/4 | F(T) | 6.58 | 7.54 | 8.612 |
| α | 1.048 | 1.054 | 1.046 | |
| 80/20/0/5/4 | F(T) | 6.905 | 7.754 | 8.778 |
| α | 1.158 | 1.209 | 1.242 | |
| 80/20/5/5/4 | F(T) | 6.682 | 7.8 | 8.894 |
| α | 1.091 | 1.084 | 1.074 | |
| PP/LDPE/PP-g-MA/PE-g-MA/Clay | Kissinger Method | R2 |
|---|---|---|
| ∆E/kJ·mol−1 | ||
| 100/0/0/0/0 | 22.5 | 0.999 |
| 96/0/0/0/4 | 16.7 | 0.998 |
| 91/0/5/0/4 | 18.8 | 0.993 |
| 80/20/0/0/0 | 17.2 | 0.991 |
| 80/20/0/0/4 | 15.3 | 0.988 |
| 80/20/5/0/4 | 23.5 | 0.996 |
| 80/20/0/5/4 | 27.0 | 0.991 |
| 80/20/5/5/4 | 24.7 | 0.989 |
| PP/LDPE/PP-g-MA/PE-g-MA/Clay | Spherulite Growth Rate (G), (µm/min) | To/min | R2 |
|---|---|---|---|
| 100/0/0/0/0 | 3.23 | 2 | 0.998 |
| 96/0/0/0/4 | 2.74 | 2 | 0.995 |
| 91/0/5/0/4 | 2.14 | 2 | 0.992 |
| 80/20/0/0/0 | 2.93 | 2 | 0.994 |
| 80/20/0/0/4 | 2.65 | 3 | 0.997 |
| 80/20/5/0/4 | 2.34 | 7 | 0.997 |
| 80/20/0/5/4 | 2.41 | 4 | 0.997 |
| 80/20/5/5/4 | 2.39 | 3 | 0.997 |
| PP/LDPE/PP-g-MA/PE-g-MA/Clay | Activation Energy (Ea)/kJ·mol−1 |
|---|---|
| 100/0/0/0/0 | 42.1 |
| 96/0/0/0/4 | 66.7 |
| 91/0/5/0/4 | 83.6 |
| 0/100/0/0 | 139.4 |
| 0/96/0/0/4 | 68.4 |
| 80/20/0/0/0 | 129.9 |
| 80/20/0/0/4 | 163.6 |
| 80/20/5/0/4 | 111.7 |
| 80/20/0/5/4 | 84.2 |
| 80/20/5/5/4 | 160.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofokeng, T.G.; Ray, S.S.; Ojijo, V. Influence of Selectively Localised Nanoclay Particles on Non-Isothermal Crystallisation and Degradation Behaviour of PP/LDPE Blend Composites. Polymers 2018, 10, 245. https://doi.org/10.3390/polym10030245
Mofokeng TG, Ray SS, Ojijo V. Influence of Selectively Localised Nanoclay Particles on Non-Isothermal Crystallisation and Degradation Behaviour of PP/LDPE Blend Composites. Polymers. 2018; 10(3):245. https://doi.org/10.3390/polym10030245
Chicago/Turabian StyleMofokeng, Tladi Gideon, Suprakas Sinha Ray, and Vincent Ojijo. 2018. "Influence of Selectively Localised Nanoclay Particles on Non-Isothermal Crystallisation and Degradation Behaviour of PP/LDPE Blend Composites" Polymers 10, no. 3: 245. https://doi.org/10.3390/polym10030245
APA StyleMofokeng, T. G., Ray, S. S., & Ojijo, V. (2018). Influence of Selectively Localised Nanoclay Particles on Non-Isothermal Crystallisation and Degradation Behaviour of PP/LDPE Blend Composites. Polymers, 10(3), 245. https://doi.org/10.3390/polym10030245