Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance
Abstract
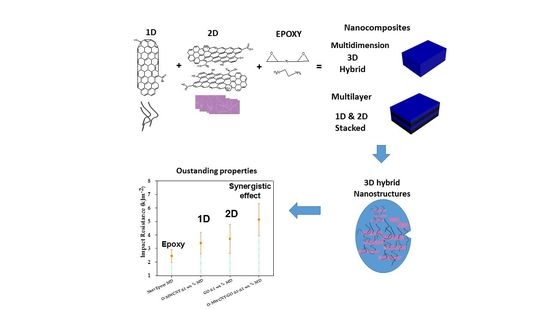
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Graphite Oxide Synthesis
2.3. Graphene Oxide Preparation
2.4. Reduced Graphene Oxide
2.5. Functionalization of CNT
2.6. Synthesis of Nanocomposites
2.7. Characterization of Carbon Nanotubes
2.8. Characterization of Nanocomposites
3. Results and Discussion
3.1. Infrared and Raman Spectroscopies of 1D and 2D Nanostructures
3.2. Transmission Electron Microscopy of Carbon Nanostructures
3.3. Infrared Spectroscopy of Nanocomposites
3.4. Impact Properties
3.5. Microstructure of 1D–2D Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials—A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef] [PubMed]
- Shtein, M.; Navid, R.; Lachman, N.; Wagner, H.; Regev, O. Fracture behavior of nanotube–polymer composites: Insights on surface roughness and failure mechanism. Compos. Sci. Technol. 2013, 87, 157–163. [Google Scholar] [CrossRef]
- Gu, J.W.; Zhang, Q.Y.; Li, H.C.; Tang, Y.S.; Kong, J.; Dang, J. Study on preparation of SiO2/epoxy resin hybrid materials by means Sol-Gel. Polym. Plast. Technol. Eng. 2007, 46, 1129–1134. [Google Scholar] [CrossRef]
- Gu, J.; Liang, C.; Zhao, X.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.Y. Highly thermally conductive flame- retardant epoxy nano composites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Sato, N.; Tölle, F.; Mülhaupt, R.; Fiedler, B.; Schulte, K. Fracture toughness and failure mechanism of graphene based epoxy composites. Compos. Sci. Technol. 2014, 97, 90–99. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Bortz, D.R.; Garcia-Heras, E.; Martin-Gullon, I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules 2013, 45, 238–245. [Google Scholar] [CrossRef]
- Subhani, T.; Latif, M.; Ahmad, I.; Rakha, S.A.; Ali, N.; Khurram, A.A. Mechanical performance of epoxy matrix hybrid nanocomposites mechanical performance of epoxy matrix hybrid nanocomposites. Mater. Des. 2015, 87, 436–444. [Google Scholar] [CrossRef]
- Bindu Sharmila, T.K.; Nair, A.B.; Abraham, B.T.; Sabura Beegum, P.M.; Thachil, E.T. Microwave exfoliated reduced graphene oxide epoxy nanocomposites for high performance applications. Polymer 2014, 55, 3614–3627. [Google Scholar] [CrossRef]
- Shen, X.; Pei, X.-Q.; Liu, Y.; Fu, S.-Y. Tribological performance of carbon nanotube–graphene oxide hybrid/epoxy composites. Compos. B 2014, 57, 120–125. [Google Scholar] [CrossRef]
- Ma, P.-C.; Liu, M.-Y.; Zhang, H.; Wang, S.-Q.; Wang, R.; Wang, K.; Wang, K.; Wong, Y.-K.; Tang, B.-Z.; Hong, S.-H.; et al. Enhanced electrical conductivity of nanocomposites containing hybrid fillers of carbon nanotubes and carbon black. ACS Appl. Mater. Interfaces 2009, 1, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Duguay, A.J.; Nader, J.W.; Kiziltas, A.; Gardner, D.J.; Dagher, H.J. Exfoliated graphite nanoplatelet-filled impact modified polypropylene nanocomposites: Influence of particle diameter, filler loading, and coupling agent on the mechanical properties. Appl. Nanosci. 2014, 4, 279–291. [Google Scholar] [CrossRef]
- Liang, C.; Song, P.; Gu, H.; Ma, C.; Guo, Y.; Zhang, H.; Xu, X.; Zhang, Q.; Gu, J. Nanopolydopamine coupled fluorescent nanozinc oxide reinforced epoxy nanocomposites. Compos. A 2017, 102, 126–136. [Google Scholar] [CrossRef]
- He, Y.; Yang, S.; Liu, H.; Shao, Q.; Chen, Q.; Lu, C.; Jiang, Y.; Liu, C.; Guo, Z. Reinforced carbon fiber laminates with oriented carbon nanotube epoxy nanocomposites: Magnetic field assisted alignment and cryogenic temperature mechanical properties. J. Colloid Interface Sci. 2018, 517, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.; Mäder, E. Health monitoring in continuous glass fibre reinforced thermoplastics: Manufacturing and application of interphase sensors based on carbon nanotubes. Compos. Sci. Technol. 2010, 70, 1589–1596. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Marco, C.; Fatou, A.G.; Ellis, G.J. Multiscale fiber-reinforced thermoplastic composites incorporating carbon nanotubes: A review. Curr. Opin. Solid State Mater. Sci. 2014, 18, 62–80. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites by chemically modified graphene via in-situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018. [Google Scholar] [CrossRef]
- Yan, X.; Gu, J.; Zheng, G.; Guo, J.; Galaska, A.M.; Yu, J.; Khan, M.A.; Sun, L.; Young, D.P.; Zhang, Q.; et al. Lowly loaded carbon nanotubes induced high electrical conductivity and giant magnetoresistance in ethylene/1-octene copolymers. Polymer 2016, 103, 315–327. [Google Scholar] [CrossRef]
- Szeluga, U.; Kumanek, B.; Trzebicka, B. Synergy in hybrid polymer/nanocarbon composites: A review. Compos. A Appl. Sci. Manuf. 2015, 73, 204–231. [Google Scholar] [CrossRef]
- Zhang, J.X.; Liang, Y.X.; Wang, X.; Zhou, H.J.; Li, S.Y.; Zhang, J.; Feng, Y.; Wang, Q.; Guo, Z. Strengthened epoxy resin with hyperbranched polyamine-ester anchored graphene oxide via novel phase transfer approach. Adv. Compos. Hybrid Mater. 2017, 1–10. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Bernal, M.M.; Hernandez, M.; Verdejo, R.; Lopez-Manchado, M.A. Comparison of filler percolation and mechanical properties in graphene and carbon nanotubes filled epoxy nanocomposites. Eur. Polym. J. 2013, 49, 1347–1353. [Google Scholar] [CrossRef]
- Ma, P.-C.; Kim, J.-K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy composites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef]
- Domun, N.; Paton, K.R.; Hadavinia, H.; Sainsbury, T.; Zhang, T.; Mohamud, H. Enhancement of fracture toughness of epoxy nanocomposites by combining nanotubes and nanosheets as fillers. Materials 2017, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, M.; Li, J.; Yu, J.; Sun, S.; Ge, S.; Guo, X.; Xie, F.; Jiang, B.; Wucjcik, E.K.; et al. Silver nanoparticles/graphene oxide decorated carbon fiber synergistic reinforcement in epoxy-based composites. Polymer 2017, 131, 263–271. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, S.; Chen, L.; Jiang, D.; Shao, Q.; Zhang, B.; Zhai, Z.; Zhang, M.; Guo, Z. Electrically insulated epoxy nanocomposites reinforced with synergistic core–shell SiO2@MWCNTs and montmorillonite bifillers. Macromol. Chem. Phys. 2017, 218, 1700357–1700366. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.; Zheng, G.; Liu, X.; Li, T.; Yan, C.; Cheng, C.; Dai, K.; Liu, C.; Changyu, S.; et al. Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C 2018. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Faiella, G.; Prado, L.A.S.A.; Tölle, F.; Müllhapt, R.; Schulte, K. Thermally reduced graphene oxide acting as a trap for multiwall carbon nanotubes in bi-filler epoxy composites. Compos. A Appl. Sci. Manuf. 2013, 49, 51–57. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, W.; Jiao, W.; Hao, L.; Yang, F. Attapulgite-graphene oxide hybrids as thermal and mechanical reinforcements for epoxy composites. Compos. Sci. Technol. 2013, 87, 29–35. [Google Scholar] [CrossRef]
- Ashby, M.F.; Ferreira, P.J.; Schodek, D.L. Classes and fundamentals. In Nanomaterials, Nanotechnologies and Design an Introduction for Engineers and Architects, 1st ed.; Butterworth–Heinemann: Oxford, UK, 2009. [Google Scholar]
- Belenkov, E.A.; Greshnyakov, V.A. Classification of structural modifications of carbon. Phys. Solid State 2013, 55, 1754–1764. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Ling, X.; Wei, Y.; Zou, L.; Xu, S. The effect of different order of purification treatments on the purity of multiwalled carbon nanotubes. Appl. Surf. Sci. 2013, 276, 159–166. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, C.; Zhao, F.; Wang, S.; Yu, L.; Zhang, D. Fabrication of a graphene/C60 nanohybrid via γ-cyclodextrin host–guest chemistry for photodynamic and photothermal therapy. Nanoscale 2017, 9, 8825–8833. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-S.; Jie, K.; Gu, J.-W.; Liang, G. Reinforced cyanate ester resins with carbon nanotubes: Surface modification, reaction activity and mechanical properties analyses. Polym. Plast. Technol. Eng. 2009, 48, 359–366. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, E.; Ramirez-Fuentes, R.; Martinez-Hernandez, A.L.; Millan-Chiu, B.; Lopez-Marin, L.M.; Castaño, V.M.; Velasco-Santos, C. Graphene oxide and reduced graphene oxide modification with polypeptide chains from chicken feather keratin. J. Alloys Compd. 2015, 643, S137–S143. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- De la Luz-Asunción, M.; Sánchez-Mendieta, V.; Martínez-Hernández, A.L.; Castaño, V.M.; Velasco-Santos, C. Adsorption of phenol from aqueous solutions by carbon nanomaterials of one and two dimensions: Kinetic and equilibrium studies. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Stobinsky, L.; Lesiak, B.; Köver, L.; Tóth, J.; Biniak, S.; Trykowski, G.; Judek, J. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectoscopy methods. J. Alloys Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rhasidi, A.M.; Shariaty-Niasar, M. Effects of surface modification on the dispersion and thermal conductivity of CNT/water nanofluids. Int. Commun. Heat Mass 2014, 54, 1–7. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martínez-Hernández, A.L.; Castaño, V.M.; Rivera-Armenta, J.L.; Medellín-Rodríguez, F.; Velasco-Santos, C. Nylon 6,6 electrospun fibres reinforced by amino functionalized 1D and 2D carbon. Mater. Sci. Eng. C 2012, 40. [Google Scholar] [CrossRef]
- Dashairya, L.; Rout, M.; Saha, P. Reduced graphene oxide-coated cotton as an efficient absorbent in oil-water separation. Adv. Compos. Hybrid Mater. 2018, 1, 135–148. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Puglia, D.; Valentini, L.; Armentano, I.; Kenny, J.M. Effects of single-walled carbon nanotube incorporation on the cure reaction of epoxy resin and its detection by Raman spectroscopy. Diam. Relat. Mater. 2003, 12, 827–832. [Google Scholar] [CrossRef]
- Mahanandia, P.; Vishwakarma, P.N.; Nanda, K.K.; Prasad, V.; Barai, K.; Mondal, A.K.; Sarangi, S.; Dey, G.K.; Subramanyam, S.V. Synthesis of multi-wall carbon nanotubes by simple pyrolysis. Solid State Commun. 2008, 145, 143–148. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Abouelsayed, A.; Anis, B.; Hassaballa, S.; Khalil, A.S.G.; Rashed, U.M.; Eid, K.A.; Al-Ashkar, E.; El hotaby, W. Preparation, characterization, Raman, and terahertz spectroscopy study on carbon nanotubes, graphene nano-sheets, and onion like carbon materials. Mater. Mater. Chem. Phys. 2017, 189, 127–135. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Abdullah, A.H.; Zainal Abidin, A.S.; Yusoh, K. Application of graphene from exfoliation in kitchen mixer allows mechanical reinforcement of PVA/graphene film. Appl. Nanosci. 2017, 7, 317–324. [Google Scholar] [CrossRef]
- He, R.; Pei, X.; Pan, L.; Tian, L.A.; Luo, F.; Sui, L.; Wan, Q.; Wang, J. Effects of ultrasonic radiation intensity on the oxidation of single walled carbon nanotubes in a mixture of sulfuric and nittric acids. NANO 2013, 8, 1350040–1350049. [Google Scholar] [CrossRef]
- Ghaleb, Z.A.; Mariatti, M.; Ariff, Z.M. Properties of graphene nanopowder and multi-walled carbon nanotube-filled epoxy thin-film nanocomposites for electronic applications: The effect of sonication time and filler loading. Compos. A Appl. Sci. Manuf. 2014, 58, 77–83. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide-carbon nanotube hybrid/epoxy composite. Carbon 2012, 5, 5429–5440. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Ed.; Jon Wiley & Sons Ltd.: West Sussex, UK, 2004; ISBN 0-470-85427-8. [Google Scholar]
- Li, Z.; Young, R.J.; Wang, R.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. The role of functional groups on graphene oxide in epoxy nanocomposites. Polymer 2013, 54, 5821–5829. [Google Scholar] [CrossRef]
- Olowojoba, G.B.; Eslava, S.; Gutierrez, E.S.; Kinloch, A.J.; Mattevi, C.; Rocha, V.G.; Taylor, A.C. In situ thermally reduced graphene oxide/epoxy composites: Thermal and mechanical properties. Appl. Nanosci. 2016, 6, 1015–1022. [Google Scholar] [CrossRef]
- Yousefi, N.; Lin, X.; Zheng, Q.; Shen, X.; Pothnis, J.R.; Jia, J.; Zussman, E.; Kim, J.-K. Simultaneous in situ reduction, self-aligment and covalent bonding in graphene oxide/epoxy composites. Carbon 2013, 59, 406–417. [Google Scholar] [CrossRef]
- Chang, H.-P.; Liu, H.-C.; Tan, C.-S. Using supercritical CO2-assisted mixing to prepare graphene/carbon nanotube/epoxy nanocomposites. Polymer 2015, 75, 125–133. [Google Scholar] [CrossRef]
- Alishahi, E.; Shadlou, S.; Doagou-R, S.; Ayatollahi, M.R. Effects of carbon nanoreinforcements of different shapes on the mechanical properties of epoxy-based nanocomposites. Macromol. Mater. Eng. 2012, 298, 670–678. [Google Scholar] [CrossRef]
- Bickford, M. Characterization and Analysis of Polymers; Jonh Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 330–356. ISBN 0470233001. [Google Scholar]
- Hassanabadi, H.M.; Rodrigue, D. Effect of particle size and shape on the reinforcing efficiency of nanoparticles in polymer nanocomposites. Macromol. Mater. Eng. 2014, 299, 1220–1231. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Yousefi, A.A.; Naderi, G. Effect of type and aspect ratio of different carbon nanotubes on cure behavior of epoxy-based nanocomposites. IRAN Polym. J. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.-Y.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, V.K.; Prakash, R. Influences of carbon nanofillers on mechanical performance of epoxy resin polymer. Appl. Nanosci. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, W.; Li, X.; Zhu, J.; Wang, H.; Zhang, L. Design and fabrication of a graphene/carbon nanotubes/activated carbon hybrid and its application for capacitive deionization. RSC Adv. 2016, 6, 5817–5823. [Google Scholar] [CrossRef]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zlozczower, I. Epoxy composites with carbon nanotubes and graphene nanopletelets-Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lin, W.-N.; Huang, Y.-L.; Tien, H.-W.; Wang, J.-Y.; Ma, C.-C.M.; Li, S.-M.; Wang, Y.-S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Ramezani, M.; Fathi, M.; Mahboubi, F. Facile synthesis of ternary MnO2/graphene nanosheets/carbon nanotubes composites with high rate capability for supercapacitor applications. Electrochim. Acta 2015, 174, 345–355. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, B.; Gao, R.; Su, G.; Liu, W.; Shi, L.; Xia, C.; Cao, L. A three-dimensional graphene-TiO2 nanotube nanocomposite with exceptional photocatalytic activity for dye degradation. Appl. Surf. Sci. 2015, 351, 303–308. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Yuan, H.; Liu, H.; Liu, C.; Li, T.; Yan, C.; Yan, X. Non-covalently functionalized graphene strengthened poly(vinyl alcohol). Mater. Des. 2018, 139, 372–379. [Google Scholar] [CrossRef]








| Neat Epoxy | |||||
|---|---|---|---|---|---|
| MWCNT 0.1 wt % | MWCNT 0.5 wt % | MWCNT-GO 0.1–0.1 wt % | MWCNT-RGO 0.1–0.1 wt % | O-MWCNT-GO 0.1–0.1 wt % | O-MWCNT-RGO 0.1–0.1 wt % |
| O-MWNTC 0.1 wt % | O-MWNTC 0.5 wt % | MWCNT-GO 0.1–0.5 wt % | MWCNT-RGO 0.1–0.5 wt % | O-MWCNT-GO 0.1–0.5 wt % | O-MWCNT-RGO 0.1–0.5 wt % |
| GO 0.1 wt % | GO 0.5 wt % | MWCNT-GO 0.5–0.1 wt % | MWCNT-RGO 0.5–0.1 wt % | O-MWCNT-GO 0.5–0.1 wt % | O-MWCNT-RGO 0.5–0.1 wt % |
| RGO 0.1 wt % | RGO 0.5 wt % | MWCNT-GO 0.5–0.5 wt % | MWCNT-RGO 0.5–0.5 wt % | O-MWCNT-GO 0.5-0.5 wt % | O-MWCNT-RGO 0.5–0.5 wt % |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Barroso, J.; Martínez-Hernández, A.L.; Rivera-Armenta, J.L.; Velasco-Santos, C. Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance. Polymers 2018, 10, 281. https://doi.org/10.3390/polym10030281
López-Barroso J, Martínez-Hernández AL, Rivera-Armenta JL, Velasco-Santos C. Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance. Polymers. 2018; 10(3):281. https://doi.org/10.3390/polym10030281
Chicago/Turabian StyleLópez-Barroso, Juventino, Ana Laura Martínez-Hernández, José Luis Rivera-Armenta, and Carlos Velasco-Santos. 2018. "Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance" Polymers 10, no. 3: 281. https://doi.org/10.3390/polym10030281
APA StyleLópez-Barroso, J., Martínez-Hernández, A. L., Rivera-Armenta, J. L., & Velasco-Santos, C. (2018). Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance. Polymers, 10(3), 281. https://doi.org/10.3390/polym10030281