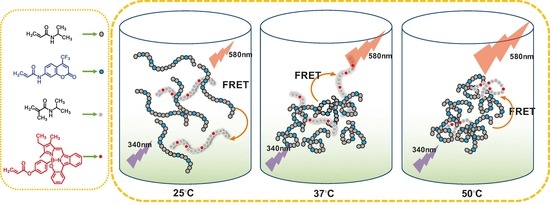
Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic of BOBPYBX
2.3. Synthesis of Fluorescent Polymers
2.4. Analytical Techniques
2.5. Cell Culture
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bai, T.; Gu, N. Micro/nanoscale thermometry for cellular thermal sensing. Small 2016, 12, 4590–4610. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Gota, C. Luminescent molecular thermometers for the ratiometric sensing of intracellular temperature. Rev. Anal. Chem. 2017, 36. [Google Scholar] [CrossRef]
- Zhou, J.; Mishra, K.; Bhagat, V.; Joy, A.; Becker, M.L. Thermoresponsive dual emission nanosensor based on quantum dots and dye labeled poly(N-isopropylacrylamide). Polym. Chem. 2015, 6, 2813–2816. [Google Scholar] [CrossRef]
- Liu, Y.; Oda, H.; Inoue, Y.; Ishihara, K. Movement of a quantum dot covered with cytocompatible and PH-responsible phospholipid polymer chains under a cellular environment. Biomacromolecules 2016, 17, 3986–3994. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Liu, T.; Zhang, G.; Liu, S. Intracellular cascade fret for temperature imaging of living cells with polymeric ratiometric fluorescent thermometers. ACS Appl. Mater. Interfaces 2015, 7, 15551–15560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, K.Y.; Tong, X.; Liu, Y.; Hu, C.; Liu, S.; Yu, Q.; Zhao, Q.; Huang, W. Phosphorescent polymeric thermometers for in vitro and in vivo temperature sensing with minimized background interference. Adv. Funct. Mater. 2016, 26, 4386–4396. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, Y.; Lee, D.; Jang, W.D. Thermoresponsive polymer and fluorescent dye hybrids for tunable multicolor emission. Adv. Mater. 2016, 28, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; He, S.; Qu, J.; Wu, H.; Wu, S.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Thermoresponsive aie polymers with fine-tuned response temperature. J. Mater. Chem. C 2016, 4, 2964–2970. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, F.; Wang, X.; Yan, H.; Song, J.; Ye, Q.; Tang, B.Z.; Xu, J. Aggregation induced emission based fluorescence PH and temperature sensors: Probing polymer interactions in poly(N-isopropyl acrylamide-co-tetra(phenyl)ethene acrylate)/poly(methacrylic acid) interpenetrating polymer networks. J. Mater. Chem. C 2015, 3, 5490–5498. [Google Scholar] [CrossRef]
- Maji, S.; Cesur, B.; Zhang, Z.; De Geest, B.G.; Hoogenboom, R. Poly(N-isopropylacrylamide) coated gold nanoparticles as colourimetric temperature and salt sensors. Polym. Chem. 2016, 7, 1705–1710. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Phan, V.H.; Lee, D.S. Stimuli-sensitive injectable hydrogels based on polysaccharides and their biomedical applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Cao, X. Affinity precipitation of cellulase using PH-response polymer with cibacron blue F3GA. Sep. Purif. Technol. 2013, 102, 136–141. [Google Scholar] [CrossRef]
- Ding, Z.; Cao, X. Affinity precipitation of human serum albumin using a thermo-response polymer with an l-thyroxin ligand. BMC Biotechnol. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kang, L.; Liu, J.; Zhang, X.; Cao, X. Preparation of PH-responsive metal chelate affinity polymer for adsorption and desorption of insulin. J. Chem. Technol. Biotechnol. 2017, 92, 1590–1595. [Google Scholar] [CrossRef]
- Lyon, L.A.; Meng, Z.; Singh, N.; Sorrell, C.D.; St John, A. Thermoresponsive microgel-based materials. Chem. Soc. Rev. 2009, 38, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qi, C.; Cheng, C.; Yang, Z.; Cao, H.; Yang, Z.; Tong, J.; Yao, X.; Lei, Z. Aie-active tetraphenylethylene cross-linked N-isopropylacrylamide polymer: A long-term fluorescent cellular tracker. ACS Appl. Mater. Interfaces 2016, 8, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Hiruta, Y.; Wang, J.; Ayano, E.; Kanazawa, H. Design of environmentally responsive fluorescent polymer probes for cellular imaging. Biomacromolecules 2015, 16, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bui, H.T.; Vales, T.P.; Cho, S.; Kim, H.-J. Multi-color fluorescence of pnipam-based nanogels modulated by dual stimuli-responsive fret processes. Dyes Pigm. 2017, 145, 216–221. [Google Scholar] [CrossRef]
- Qiao, J.; Hwang, Y.-H.; Chen, C.-F.; Qi, L.; Dong, P.; Mu, X.-Y.; Kim, D.-P. Ratiometric fluorescent polymeric thermometer for thermogenesis investigation in living cells. Anal. Chem. 2015, 87, 10535–10541. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.T. A pnipam-based fluorescent nanothermometer with ratiometric readout. Chem. Commun. 2011, 47, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, C.; Wang, D.; Liu, S. Fret-derived ratiometric fluorescent k+ sensors fabricated from thermoresponsive poly(N-isopropylacrylamide) microgels labeled with crown ether moieties. J. Phys. Chem. B 2010, 114, 12213–12220. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Iwai, K.; Matsumura, Y.; Uchiyama, S.; de Silva, A.P. Development of fluorescent microgel thermometers based on thermo-responsive polymers and their modulation of sensitivity range. J. Mater. Chem. 2005, 15, 2796–2800. [Google Scholar] [CrossRef]
- Bonnett, R.; McManus, K.A. Approaches to the stepwise synthesis of benzoporphyrins and phthalocyanines. Part 1. Synthesis of opp-dibenzoporphyrins (dibenzo [g,q] porphyrins). J. Chem. Soc. Perkin Trans. 1 1996, 2461–2466. [Google Scholar] [CrossRef]
- Diana, P.; Martorana, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Cirrincione, G. Nucleophilic substitutions in the isoindole series as a valuable tool to synthesize derivatives with antitumor activity. Tetrahedron 2011, 67, 2072–2080. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, W.; Chen, S.; Wu, Q.; Yu, C.; Wei, Y.; Xu, Y.; Hao, E.; Jiao, L. Sterically protected N2O-type benzopyrromethene boron complexes from boronic acids with intense red/near-infrared fluorescence. Org. Lett. 2017, 19, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, P.; Chen, H.; Wang, C. Amine-accelerated manganese-catalyzed aromatic C–H conjugate addition to α,β-unsaturated carbonyls. Chem. Commun. 2014, 50, 14558–14561. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zheng, X.; Li, S.; Cao, X. Immobilization of cellulase onto a recyclable thermo-responsive polymer as bioconjugate. J. Mol. Catal. B Enzym. 2016, 128, 39–45. [Google Scholar] [CrossRef]






| Polymers | Mn | Mw | PDI |
|---|---|---|---|
| PNB | 5957 | 7954 | 1.33121 |
| PNmR | 11,144 | 16,140 | 1.44831 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Wang, C.; Feng, G.; Zhang, X. Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET. Polymers 2018, 10, 283. https://doi.org/10.3390/polym10030283
Ding Z, Wang C, Feng G, Zhang X. Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET. Polymers. 2018; 10(3):283. https://doi.org/10.3390/polym10030283
Chicago/Turabian StyleDing, Zhaoyang, Chunfei Wang, Gang Feng, and Xuanjun Zhang. 2018. "Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET" Polymers 10, no. 3: 283. https://doi.org/10.3390/polym10030283
APA StyleDing, Z., Wang, C., Feng, G., & Zhang, X. (2018). Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET. Polymers, 10(3), 283. https://doi.org/10.3390/polym10030283