Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
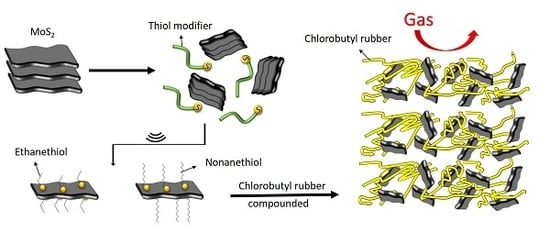
2.2. Exfoliation of MoS2
2.3. Preparation of MoS2-butyl Rubber Nanocomposites
2.4. Characterization
3. Results and Discussion
3.1. Exfoliation of MoS2
3.2. Characterization of MoS2-butyl rubber Nanocomposites
3.3. Tensile Properties of MoS2-butyl Rubber Nanocomposites
3.4. Dynamic Mechanical Analysis of MoS2-butyl Rubber Nanocomposites
3.5. Gas Barrier Properties of MoS2-butyl Rubber Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoon, Y.; Ganapathi, K.; Salahuddin, S. How good can monolayer MoS2 transistors be? Nano Lett. 2011, 11, 3768–3773. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, R.; Su, Y.-A.; Tsai, H.-C.; Jeng, R.-J. MoS2-Gd chelate magnetic nanomaterials with core-shell structure used as contrast agents in in vivo magnetic resonance imaging. ACS Appl. Mater. Interfaces 2016, 8, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wen, P.; Cheng, Y.; Liu, L.; Tai, Q.; Hu, Y.; Liew, K.M. Defect-free MoS2 nanosheets: Advanced nanofillers for polymer nanocomposites. Compos. Part A 2016, 81, 61–68. [Google Scholar] [CrossRef]
- Paul, D.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Zachariah, A.K.; Geethamma, V.; Chandra, A.K.; Mohammed, P.; Thomas, S. Rheological behaviour of clay incorporated natural rubber and chlorobutyl rubber nanocomposites. RSC Adv. 2014, 4, 58047–58058. [Google Scholar] [CrossRef]
- Liu, T.; Tjiu, W.C.; Tong, Y.; He, C.; Goh, S.S.; Chung, T.S. Morphology and fracture behavior of intercalated epoxy/clay nanocomposites. J. Appl. Polym. Sci. 2004, 94, 1236–1244. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, W.; Li, Z.; Wang, Y.; Wu, Y.; Zhang, L. A new strategy to improve the gas barrier property of isobutylene-isoprene rubber/clay nanocomposites. Polym. Test. 2008, 27, 270–276. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Peng, Z.; Zhang, Y. Influence of the clay modification and compatibilizer on the structure and mechanical properties of ethylene-propylene-diene rubber/montmorillonite composites. J. Appl. Polym. Sci. 2004, 92, 638–646. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; George, G.; Motta, N.; Waclawik, E.; Yan, C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J. Appl. Phys. 2014, 116, 053518. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Li, H.; Wu, S.; Liu, Y.; Zheng, J. Enhanced mechanical and gas barrier properties of rubber nanocomposites with surface functionalized graphene oxide at low content. Polymer 2013, 54, 1930–1937. [Google Scholar] [CrossRef]
- Lian, H.; Li, S.; Liu, K.; Xu, L.; Wang, K.; Guo, W. Study on modified graphene/butyl rubber nanocomposites. I. Preparation and characterization. Polym. Eng. Sci. 2011, 51, 2254–2260. [Google Scholar] [CrossRef]
- Fan, X.; Khosravi, F.; Rahneshin, V.; Shanmugam, M.; Loeian, M.; Jasinski, J.; Cohn, R.W.; Terentjev, E.; Panchapakesan, B. MoS2 actuators: Reversible mechanical responses of MoS2-polymer nanocomposites to photons. Nanotechnology 2015, 26, 261001. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.P.; Miller, A.C.; Spontak, R.J. Highly CO2-permeable and selective polymer nanocomposite membranes. Adv. Mater. 2003, 15, 729–733. [Google Scholar] [CrossRef]
- Ma, G.; Peng, H.; Mu, J.; Huang, H.; Zhou, X.; Lei, Z. In situ intercalative polymerization of pyrrole in graphene analogue of MoS2 as advanced electrode material in supercapacitor. J. Power Sources 2013, 229, 72–78. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, R.; Zhuo, J.; Zhu, Z.; Shao, Y.; Li, M. Direct detection of DNA below ppb level based on thionin-functionalized layered MoS2 electrochemical sensors. Anal. Chem. 2014, 86, 12064–12069. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.S.; De, M.; Kim, J.; Byun, S.; Dykstra, C.; Yu, J.; Huang, J.; Dravid, V.P. Ligand conjugation of chemically exfoliated MoS2. J. Am. Chem. Soc. 2013, 135, 4584–4587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.-C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Dungey, K.E.; Curtis, M.D.; Penner-Hahn, J.E. Structural characterization and thermal stability of MoS2 intercalation compounds. Chem. Mater. 1998, 10, 2152–2161. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, S.; Liu, S.; Cai, Y.; Low, M.; Teng, C.P.; Phang, I.Y.; Cheng, Y.; Duei, K.L.; Srinivasan, B.M. Protein induces layer-by-layer exfoliation of transition metal dichalcogenides. J. Am. Chem. Soc. 2015, 137, 6152–6155. [Google Scholar] [CrossRef] [PubMed]
- Bazylewski, P.; Divigalpitiya, R.; Fanchini, G. In situ raman spectroscopy distinguishes between reversible and irreversible thiol modifications in l-cysteine. RSC Adv. 2017, 7, 2964–2970. [Google Scholar] [CrossRef]
- Marshall, C.P.; Marshall, A.O. The potential of raman spectroscopy for the analysis of diagenetically transformed carotenoids. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2010, 368, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.M.; Kim, M.; Yim, S.; Choi, M.-J.; Choi, J.; Yoo, S.; Jung, Y.S. Controlled doping of vacancy-containing few-layer MoS2 via highly stable thiol-based molecular chemisorption. ACS Nano 2015, 9, 12115–12123. [Google Scholar] [CrossRef] [PubMed]
- Biswas, Y.; Dule, M.; Mandal, T.K. Poly(ionic liquid)-promoted solvent-borne efficient exfoliation of MoS2/MoSe2 nanosheets for dual-responsive dispersion and polymer nanocomposites. J. Phys. Chem. C 2017, 121, 4747–4759. [Google Scholar] [CrossRef]
- Triantafillidis, C.S.; LeBaron, P.C.; Pinnavaia, T.J. Homostructured mixed inorganic-organic ion clays: A new approach to epoxy polymer-exfoliated clay nanocomposites with a reduced organic modifier content. Chem. Mater. 2002, 14, 4088–4095. [Google Scholar] [CrossRef]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Koratkar, N. Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Choi, M.H.; Wang, K.H.; Kim, S.O.; Kim, Y.K.; Chung, I.J. Synthesis of exfoliated PMMA/Na-MMT nanocomposites via soap-free emulsion polymerization. Macromolecules 2001, 34, 8978–8985. [Google Scholar] [CrossRef]













| MoS2-ethanethiol-butyl rubber (cc/m2-day) | MoS2-nonanethiol-butyl rubber (cc/m2-day) | |
|---|---|---|
| 0 phr | 90.9 | 90.9 |
| 0.5 phr | 42.3 | 47.2 |
| 1 phr | 48.2 | 46.8 |
| 3 phr | 44.6 | 45.8 |
| 5 phr | 43.7 | 46.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-Y.; Lin, S.-Y.; Tsai, H.-C. Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties. Polymers 2018, 10, 238. https://doi.org/10.3390/polym10030238
Tsai C-Y, Lin S-Y, Tsai H-C. Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties. Polymers. 2018; 10(3):238. https://doi.org/10.3390/polym10030238
Chicago/Turabian StyleTsai, Chi-Yang, Shuian-Yin Lin, and Hsieh-Chih Tsai. 2018. "Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties" Polymers 10, no. 3: 238. https://doi.org/10.3390/polym10030238
APA StyleTsai, C. -Y., Lin, S. -Y., & Tsai, H. -C. (2018). Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties. Polymers, 10(3), 238. https://doi.org/10.3390/polym10030238