Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization
2.3. Characterization
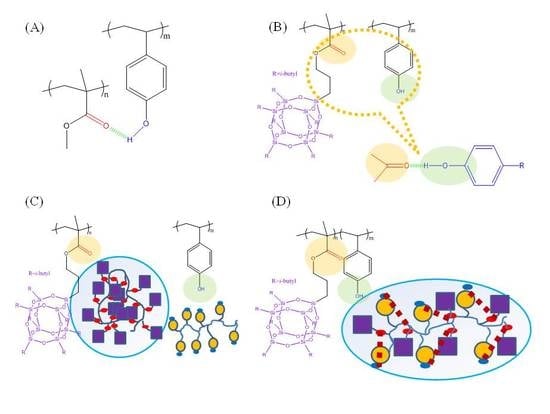
3. Results and Discussion
3.1. Synthesis of PAS-co-PMAPOSS Random Copolymers
3.2. Synthesis of PVPh-co-PMAPOSS Random Copolymers
3.3. Interaction and Phase Behavior of PVPh-co-PMAPOSS Random Copolymers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coleman, M.M.; Painter, P.C. Hydrogen bonded polymer blends. Prog. Polym. Sci. 1995, 20, 1–59. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen bonds in polymer blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen-bonding in polymer blends. J. Polym. Res. 2008, 15, 459–486. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lee, H.F.; Huang, W.J.; Jeong, K.U.; Chang, F.C. Solid state and solution self-assembly of helical polypeptides tethered to polyhedral oligomeric silsesquioxanes. Macromolecules 2009, 42, 1619–1626. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lu, F.H.; Hong, J.L.; Kuo, S.W. Strong emission of 2, 4, 6-triphenylpyridine-functionalized polytyrosine and hydrogen-bonding interactions with poly (4-vinylpyridine). Polym. Chem. 2015, 6, 6340–6350. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen bonding in polymeric materials, 1st ed.; Wiley-VCH: Weinheim, Germany, 2018; ISBN 978-3-527-34188-7. [Google Scholar]
- Abate, L.; Blanco, I.; Cicala, G.; Recca, G.; Scamporrino, A. The influence of chain-ends on the thermal and rheological properties of some 40/60 PES/PEES copolymers. Polym. Sci. Eng. 2009, 49, 1477–1483. [Google Scholar] [CrossRef]
- Zhang, X.; Takegoshi, K.; Hikichi, K. Poly(vinylphenol)/poly(methyl acrylate) and poly(vinylphenol)/poly(methyl methacrylate) blends: Hydrogen bonding, miscibility, and blending effects on molecular motions as studied by carbon-13 CP/MAS NMR. Macromolecules 1991, 24, 5756–5762. [Google Scholar] [CrossRef]
- Li, D.; Brisson, J. DMTA and FTIR Investigation of the Phase Behavior of Poly(methyl methacrylate)–Poly(4-vinylphenol) Blends. Macromolecules 1996, 29, 868–874. [Google Scholar] [CrossRef]
- Li, D.; Brisson, J. Hydrogen bonds in poly(methyl methacrylate)-poly(4-vinyl phenol) blends: 1. Quantitative analysis using FTi.r. spectroscopy. Polymer 1998, 39, 793–800. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y. FTIR and FT-Raman Studies of Partially Miscible Poly(methyl methacrylate)/Poly(4-vinylphenol) Blends in Solid States. Macromolecules 1997, 30, 286–292. [Google Scholar] [CrossRef]
- Goh, S.H.; Siow, K.S. Miscibility of poly(p-vinyl phenol) with polymethacrylates. Polym. Bull. 1987, 17, 453–457. [Google Scholar] [CrossRef]
- Serman, S.J.; Painter, P.C.; Coleman, M.M. Studies of the phase behaviour of poly(vinyl phenol)-poly(n-alkyl methacrylate) blends. Polymer 1991, 32, 1049–1058. [Google Scholar] [CrossRef]
- Lin, C.L.; Chen, W.C.; Liao, C.S.; Su, Y.C.; Huang, C.F.; Kuo, S.W.; Chang, F.C. Sequence Distribution and Polydispersity Index Affect the Hydrogen-Bonding Strength of Poly(vinylphenol-co-methyl methacrylate) Copolymers. Macromolecules 2005, 38, 6435–6444. [Google Scholar] [CrossRef]
- Painter, P.C.; Veytsman, B.; Kumar, S.; Shenoy, S.; Graf, J.F.; Xu, Y.; Coleman, M.M. Intramolecular Screening Effects in Polymer Mixtures. 1. Hydrogen-Bonded Polymer Blends. Macromolecules 1997, 30, 932–942. [Google Scholar] [CrossRef]
- Pehlert, G.J.; Painter, P.C.; Veytsman, B.; Coleman, M.M. Functional Group Accessibility in Hydrogen-Bonded Polymer Blends. 2. Miscibility Map of 2,3-Dimethylbutadiene-stat-vinylphenol Blends with Ethylene-stat-vinyl acetate. Macromolecules 1997, 30, 3671–3677. [Google Scholar] [CrossRef]
- Pehlert, G.J.; Painter, P.C.; Coleman, M.M. Functional Group Accessibility in Hydrogen-Bonded Polymer Blends. 3. Steric Shielding Effects. Macromolecules 1998, 31, 8423–8424. [Google Scholar] [CrossRef]
- Coleman, M.M.; Xu, Y.; Painter, P.C. Compositional heterogeneities in hydrogen-bonded polymer blends: infrared spectroscopic results. Macromolecules 1994, 27, 127–134. [Google Scholar] [CrossRef]
- Pruthtikul, R.; Coleman, M.; Painter, P.C.; Tan, N.B. Screening Effects in Solutions of a Hyperbranched, Dendrimer-Like Polyester. Macromolecules 2001, 34, 4145–4150. [Google Scholar] [CrossRef]
- Huang, C.F.; Kuo, S.W.; Lin, F.L.; Huang, W.J.; Wang, C.F.; Chen, W.Y.; Chang, F.C. Influence of PMMA-Chain-End Tethered Polyhedral Oligomeric Silsesquioxanes on the Miscibility and Specific Interaction with Phenolic Blends. Macromolecules 2006, 39, 300–308. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhang, W.B.; Hsieh, I.F.; Zhang, G.L.; Cao, Y.; Li, X.P.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z.D. Breaking symmetry toward nonspherical janus particles based on polyhedral oligomeric silsesquioxanes: Molecular design, “click” synthesis, and hierarchical structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Huang, K.W.; Chiou, C.W.; Kuo, S.W. Complementary Multiple Hydrogen Bonding Interactions Induce the Self-Assembly of Supramolecular Structures from Heteronucleobase-Functionalized Benzoxazine and Polyhedral Oligomeric Silsesquioxane Nanoparticles. Macromolecules 2012, 45, 9020–9028. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Hayakawa, T.; Kuo, S.W. Hydrogen bond interactions mediate hierarchical self-assembly of POSS-containing block copolymers blended with phenolic resin. Macromolecules 2014, 47, 8709–8721. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hsu, K.C.; Hong, J.L.; Kuo, S.W. Unexpected fluorescence from maleimide-containing polyhedral oligomeric silsesquioxanes: Nanoparticle and sequence distribution analyses of polystyrene-based alternating copolymers. Polym. Chem. 2016, 7, 135–145. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Jheng, Y.R.; Kuo, S.W. Unusual Emission of Polystyrene-Based Alternating Copolymers from Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxanes Nanoparticle and Sensors for Metal Ions. Polymers 2017, 9, 103. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Hayakawa, T.; Kuo, S.W. Strong Screening Effect of Polyhedral Oligoneric Silsesquioxanes (POSS) Nanoparticles on Hydrogen Bonded Polymer Blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef]
- Kuo, S.W.; Xu, H.; Huang, C.F.; Chang, F.C. Significant Glass Transition Temperature Increase in Hydrogen Bonded Copolymers. J. Polym. Sci. Part B: Polym. Phys. 2002, 40, 2313–2323. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Significant Thermal Property and Hydrogen Bonding Strength Increase in Poly(vinylphenol-co-vinylpyrrolidone) Copolymer. Polymer 2003, 44, 3021–3030. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Lin, P.Y.; Kuo, S.W. Temperature-, pH-, and CO2-Sensitive Poly(N-isopropylacryl amide-co–acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers 2016, 8, 434. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Effect of copolymer composition on the miscibility of poly(styrene-co-acetoxystyrene) with phenolic resin. Polymer 2001, 42, 9843–9848. [Google Scholar] [CrossRef]
- Xu, H.; Kuo, S.W.; Lee, J.S.; Chang, F.C. Preparations, Thermal Properties, and Tg Increase Mechanism of Inorganic/Organic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2002, 35, 8788–8793. [Google Scholar] [CrossRef]
- Kwei, T.K. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
- Waddan, A.J.; Coughlin, E.B. Crystal Structure of Polyhedral Oligomeric Silsequioxane (POSS) Nano-materials: A Study by X-ray Diffraction and Electron Microscopy. Chem. Mater. 2003, 15, 4555–4561. [Google Scholar] [CrossRef]
- Carroll, J.B.; Waddon, A.J.; Nakade, H.; Rotello, V.M. Plug and Play Polymers. Thermal and X-ray Characterizations of Noncovalently Grafted Polyhedral Oligomeric Silsesquioxane (POSS)−Polystyrene Nanocomposites. Macromolecules 2003, 36, 6289–6291. [Google Scholar] [CrossRef]













| Nomenclature | Molar Ratio | Weight Ratio | Mnb (g/mol) | PDIb | Tgc (°C) | ||
|---|---|---|---|---|---|---|---|
| Feeding | Product | Feeding | Producta | ||||
| Pure PAS | 100/0 | 100/0 | 100/0 | 100/0 | 17,700 | 1.61 | 127 |
| PAS90-co-PMAPOSS10 | 98.13/1.87 | 97.26/2.74 | 90/10 | 85.92/14.08 | 19,800 | 1.77 | 122 |
| PAS80-co-PMAPOSS20 | 95.89/4.11 | 95.7/4.3 | 80/20 | 79.28/20.72 | 24,300 | 1.53 | 117 |
| PAS70-co-PMAPOSS30 | 93.14/6.86 | 93.82/6.18 | 70/30 | 72.29/27.71 | 27,700 | 1.48 | 110 |
| PAS60-co-PMAPOSS40 | 89.72/10.28 | 91.51/8.49 | 60/40 | 64.94/35.06 | 13,700 | 1.63 | 106 |
| PAS50-co-PMAPOSS50 | 85.35/14.65 | 88.27/11.73 | 50/50 | 56.39/43.61 | 29,500 | 1.32 | 105 |
| Pure PMAPOSS | 0/100 | 0/100 | 0/100 | 0/100 | 38,600 | 1.07 | 56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Lin, R.-C.; Tseng, S.-M.; Kuo, S.-W. Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers 2018, 10, 303. https://doi.org/10.3390/polym10030303
Chen W-C, Lin R-C, Tseng S-M, Kuo S-W. Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers. 2018; 10(3):303. https://doi.org/10.3390/polym10030303
Chicago/Turabian StyleChen, Wei-Cheng, Ruey-Chorng Lin, Shih-Min Tseng, and Shiao-Wei Kuo. 2018. "Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers" Polymers 10, no. 3: 303. https://doi.org/10.3390/polym10030303
APA StyleChen, W. -C., Lin, R. -C., Tseng, S. -M., & Kuo, S. -W. (2018). Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers, 10(3), 303. https://doi.org/10.3390/polym10030303