A Highly Efficient Aromatic Amine Ligand/Copper(I) Chloride Catalyst System for the Synthesis of Poly(2,6-dimethyl-1,4-phenylene ether)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Oxidative Coupling Polymerization of PPE and Oxygen Uptake Measurements
2.3. Measurement of DPQ Content
2.4. Calculation of the Number of Moles of Oxygen Being Reacted during Polymerization Reaction
2.5. Determination of Molecular Weight of the Polymers and Dilute Solution Viscosity
3. Results and Discussion
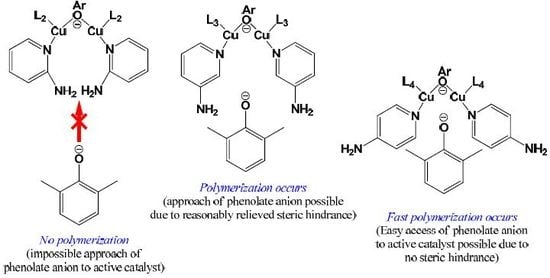
3.1. Effects of Chemical Structure of Ligand on the Rate of Polymerization
3.2. Effects of Ligand and Polymerization Conditions on DPQ Formation and Molecular Weight of Polymer
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, M. Poly(phenylene oxide). In Handbook of Engineering and Specialty Thermoplastics; Thomas, S., Visakh, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2011; Volume 3, pp. 15–53. [Google Scholar]
- Karasz, F.E.; Bair, H.E.; O’Reilly, J.M. Thermodynamic properties of poly(2,6-dimethyl-1,4-phenylene ether). J. Polym. Sci. Part B 1968, 6, 1141–1148. [Google Scholar] [CrossRef]
- Kim, D.K.; Song, K.H.; Koo, C.M.; Hong, S.M.; Chae, D.W. Characterization of compatibilized blends of nylon 66/poly(2,6-dimethyl-1,4-phenylene ether)/high-impact polystyrene filled with phosphinate-based flame retardants: Mechanical property, rheological behavior, and flame retardancy. J. Fire Sci. 2015, 33, 339–357. [Google Scholar] [CrossRef]
- Jin, K.; Torkelson, J.M. Tg and Tg breadth of poly(2,6-dimethyl-1,4-phenylene oxide)/polystyrene miscible polymer blends characterized by differential scanning calorimetry, ellipsometry, and fluorescence spectroscopy. Polymer 2015, 65, 233–242. [Google Scholar] [CrossRef]
- Lee, C.; Salehiyan, R.; Ham, D.S.; Cho, S.K.; Lee, S.J.; Kim, K.J.; Yoo, Y.; Hyun, K.; Lee, J.H.; Choi, W.J. Influence of carbon nanotubes localization and transfer on electrical conductivity in PA66/(PS/PPE)/CNTs nanocomposites. Polymer 2016, 84, 198–208. [Google Scholar] [CrossRef]
- Li, Y.F.; Meng, J.; Zou, X.D. Flame Retardant Thermoplastic Composition Useful For Laser Direct Structuring. U.S. Patent 20130289178 A1, 23 May 2008. [Google Scholar]
- Bussink, J.; van de Grampel, H.T. Poly(phenylene Oxide). In Industrial Polymers Handbook: Products, Processes, Applications, 2nd ed.; Wilks, E.S., Ed.; Wiley-VCH: Cham, Germany, 2001; Volume 3, pp. 1333–1348. ISBN 978-3527302604. [Google Scholar]
- Hay, A.S. Poly(2,6-diphenyl-1,4-phenylene oxide). Macromolecules 1969, 2, 107–108. [Google Scholar] [CrossRef]
- Saito, K.; Pant, S.; Hearn, M.T.W. Oxidative polymerization of 2,6-dimethylphenol in water using bis-triazacyclononane copper catalyst. J. Appl. Polym. Sci. 2011, 122, 2174–2180. [Google Scholar] [CrossRef]
- Aubel, P.G. Various Aspects of Copper-Catalysed Oxidative Coupling Reaction of 2,6-Dimethylphenol. Ph.D. Thesis, Universiteit Leiden, Leiden, The Netherlands, 19 June 2002. [Google Scholar]
- Baesjou, P.J.; Driessen, W.L.; Challa, G.; Reedijk, J. Ab Initio calculations on 2,6-dimethylphenol and 4-(2,6-dimethylphenoxy)-2,6-dimethylphenol. Evidence of an important role for the phenoxonium cation in the copper-catalyzed oxidative phenol coupling reaction. J. Am. Chem. Soc. 1997, 119, 12590–12594. [Google Scholar] [CrossRef]
- Gu, C.; Zhu, J.; Shentu, B.; Liu, Q.; Weng, Z. Oxidative polymerization behavior of 2,6-dimethylphenol in aqueous media with potassium ferricyanide. Chin. J. Polym. Sci. 2009, 27, 543–549. [Google Scholar] [CrossRef]
- Saito, K.; Kuwashiro, N.; Nishide, H. Catalyzed oxidative polymerization to form poly(2,6-dimethyl-1,4-phenylene oxide) in water using water-soluble copper complex. Polymer 2006, 47, 6581–6584. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.G. Carboxylic-supported copper complexes as catalyst for the green oxidative coupling of 2,6-dimethylphenol: Synthesis, characterization and structure. Comptes Rendus Chim. 2013, 16, 451–454. [Google Scholar] [CrossRef]
- Kim, N.C.; Kim, Y.T.; Nam, S.W.; Jeon, B.S.; Kim, Y.J. Synthesis of poly(2,6-dimethyl-1,4-phenylene ether) with controlled molecular weight via suspension polymerization catalyzed by amine–copper (I) complexes under various reaction conditions. Polym. Bull. 2013, 70, 23–33. [Google Scholar] [CrossRef]
- Sivek, R.; Bures, F.; Pytela, O.; Kulhanek, J. Imidazole-based Potential Bi- and Tridentate Nitrogen Ligands: Synthesis, Characterization and Application in Asymmetric Catalysis. Molecules 2008, 13, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Gert, B.; Ger, C. Studies on immobilized polymer-bound imidazole-copper (II) complexes as catalysts. 3. Immobilization of copper (II) complexes of poly(styrene-co-N-vinylimidazole) by grafting on silica and their catalysis of oxidative coupling of 2,6-disubstituted phenols. Macromolecules 1991, 24, 3982–3987. [Google Scholar] [CrossRef]
- White, D.M.; Nye, S.A. Carbon-13 NMR study of poly(2,6-dimethyl-1,4-phenylene oxide)s. Sites of amine incorporation. Macromolecules 1990, 23, 1318–1329. [Google Scholar] [CrossRef]
- Li, K.T.; Shieh, D.C. Polymerization of 2,6-dimethylphenol with mixed-ligand copper complexes. Ind. Eng. Chem. Res. 1994, 33, 1107–1112. [Google Scholar] [CrossRef]
- Chen, C.W.; Lin, I.H.; Lin, C.C.; Lin, J.L.; Horie, M. Synthesis of poly(2,6-dimethyl-1,4-phenylene oxide) derivatives in water using water-soluble copper complex catalyst with natural ligands. Polymer 2013, 54, 5684–5690. [Google Scholar] [CrossRef]
- Baesjou, P.J.; Driessen, W.L.; Challa, G.; Reedijk, J. A kinetic and spectroscopic study on the copper catalyzed oxidative coupling polymerization of 2,6-dimethylphenol. X-ray structure of the catalyst precursor tetrakis (N-methylimidazole) bis(nitrato) copper(II). J. Mol. Catal. A Chem. 1996, 110, 195–210. [Google Scholar] [CrossRef]
- Bruice, T.C.; Schmir, G.L. Imidazole Catalysis II The Reaction of substituted imidazoles with phenyl acetates in aqueous solution. J. Am. Chem. Soc. 1958, 80, 148–156. [Google Scholar] [CrossRef]
- Angyal, S.J.; Angyal, C.L. The tautomerism of N-hetero-aromatic amines. Part I. J. Chem. Soc. 1952, 1461–1466. [Google Scholar] [CrossRef]
- pKa Data Compiled by R. Williams. Available online: https://www.chem.wisc.edu/areas/reich/pkatable/pKa_compilation-1-Williams.pdf (accessed on 10 August 2017).
- Gamez, P.; Simons, C.; Steensma, R.; Driessen, W.L.; Challa, G.; Reedijk, J. A spectacular increase in the polymerization rate of 2,6-dimethylphenol induced by acetonitrile. Eur. Polym. J. 2001, 37, 1293–1296. [Google Scholar] [CrossRef]
- Liao, B.S.; Liu, Y.H.; Peng, S.M.; Liu, S.T. Efficient oxidative coupling of 2,6-disubstituted phenol catalyzed by a dicopper (II) complex. Dalton Trans. 2012, 41, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Hideyuki, H.; Kyokaishi, Y.G.K. Radical-controlled oxidative polymerization of phenols. J. Synth. Org. Chem. Jpn. 2005, 63, 970–981. [Google Scholar] [CrossRef]
- Uchidma, E.; Anko, M.; Ishide, H. The kinetics of the oxidative polymerization of 2.6-xylenol with a copper-amine complex. Makromol. Chem. 1972, 151, 221–234. [Google Scholar] [CrossRef]
- Hay, A.S. Polymerization by oxidative coupling: Discovery and commercialization of PPO® and Noryl® resins. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 505–517. [Google Scholar] [CrossRef]
- Liu, Q.; Shentu, B.; Gu, C.; Weng, Z. The initial oxidative polymerization kinetics of 2,6-dimethylphenol with a Cu–EDTA complex in water. Eur. Polym. J. 2009, 45, 1080–1085. [Google Scholar] [CrossRef]
- Kobayashi, S.; Higashimura, H. Oxidative polymerization of phenols revisited. Prog. Polym. Sci. 2003, 28, 1015–1048. [Google Scholar] [CrossRef]




| Condition | 2,6-DMP (g) | Molar ratios | ||
|---|---|---|---|---|
| [2,6-DMP]:[Ligand]:[CuCl] | ||||
| 1 | 1 | 200 | 70 | 2 |
| 2 | 200 | 35 | 2 | |
| 3 | 200 | 70 | 1 | |
| Rate of Polymerization (10-4 mol/L·s) | ||||||
|---|---|---|---|---|---|---|
| Ligand | L1 | L2 | L3 | L4 | L5 | |
| Condition |  |  |  |  |  | |
| 1 | 5.40 | X * | 6.53 | 6.98 | 3.89 | |
| 2 | 4.73 | X | 3.57 | 6.58 | X | |
| 3 | 1.68 | X | 2.38 | 2.77 | 1.51 | |
| Ligands | pKa | |
|---|---|---|
| L1 |  | 6.95 [22] |
| L2 |  | 6.86 [23] |
| L3 |  | 5.80 [23] |
| L4 |  | 8.96 [23] |
| L5 |  | 5.14 [24] |
| Condition | L1 | L3 | L4 | L5 |
|---|---|---|---|---|
| 1 | 4.3 | 4.2 | 2.2 | 4.4 |
| 2 | 10.4 | 7.7 | 2.8 | 15.7 |
| 3 | 3.3 | 3.9 | 2.5 | 5.7 |
| Ligand | Condition | Yield (%) | Intrinsic Viscosity (dL/g) | Mn | Mw | PDI |
|---|---|---|---|---|---|---|
| L1 | 1 | 74.6 | 0.63 | 12,400 | 36,800 | 2.97 |
| 2 | 75.6 | 0.60 | 8700 | 22,800 | 2.62 | |
| 3 | 69.5 | 0.20 | 8100 | 13,500 | 1.67 | |
| L2 | 1 | X * | X | X | X | X |
| 2 | X | X | X | X | X | |
| 3 | X | X | X | X | X | |
| L3 | 1 | 82.6 | 0.84 | 15,400 | 44,000 | 2.86 |
| 2 | 74.6 | 0.39 | 14,200 | 33,700 | 2.37 | |
| 3 | 96.7 | 1.02 | 16,100 | 44,500 | 2.76 | |
| L4 | 1 | 78.6 | 0.45 | 9,060 | 31,500 | 3.48 |
| 2 | 72.6 | 0.36 | 13,600 | 32,700 | 2.40 | |
| 3 | 72.6 | 0.29 | 12,700 | 29,400 | 2.31 | |
| L5 | 1 | 61.5 | 0.40 | 12,400 | 29,400 | 2.37 |
| 2 | X | X | X | X | X | |
| 3 | 64.5 | 0.59 | 14,900 | 38,900 | 2.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Shin, M.J.; Kim, Y.T.; Kim, J.-I.; Kim, Y.J. A Highly Efficient Aromatic Amine Ligand/Copper(I) Chloride Catalyst System for the Synthesis of Poly(2,6-dimethyl-1,4-phenylene ether). Polymers 2018, 10, 350. https://doi.org/10.3390/polym10040350
Kim K, Shin MJ, Kim YT, Kim J-I, Kim YJ. A Highly Efficient Aromatic Amine Ligand/Copper(I) Chloride Catalyst System for the Synthesis of Poly(2,6-dimethyl-1,4-phenylene ether). Polymers. 2018; 10(4):350. https://doi.org/10.3390/polym10040350
Chicago/Turabian StyleKim, Kisoo, Min Jae Shin, Yong Tae Kim, Joong-In Kim, and Young Jun Kim. 2018. "A Highly Efficient Aromatic Amine Ligand/Copper(I) Chloride Catalyst System for the Synthesis of Poly(2,6-dimethyl-1,4-phenylene ether)" Polymers 10, no. 4: 350. https://doi.org/10.3390/polym10040350
APA StyleKim, K., Shin, M. J., Kim, Y. T., Kim, J. -I., & Kim, Y. J. (2018). A Highly Efficient Aromatic Amine Ligand/Copper(I) Chloride Catalyst System for the Synthesis of Poly(2,6-dimethyl-1,4-phenylene ether). Polymers, 10(4), 350. https://doi.org/10.3390/polym10040350