Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems
Abstract
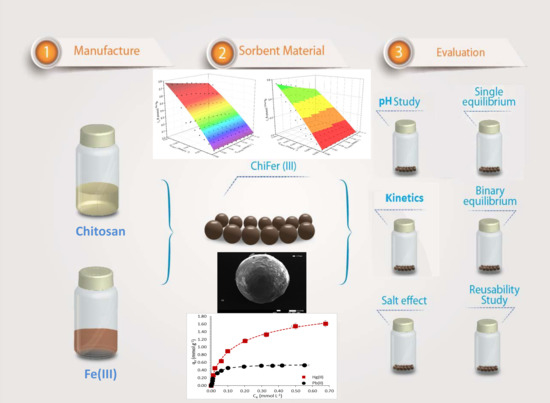
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Composite Beads
2.3. Characterization
2.4. pH Study
2.5. Equilibrium Study
2.6. Kinetics
2.7. Salt Effects
2.8. Desorption Cycles
3. Results and Discussion
3.1. Characterization
3.1.1. SEM-EDX Analysis
3.1.2. FTIR and TGA Analysis
3.2. pH Study
3.3. Equilibrium
3.4. Kinetics Studies
3.5. Salt Effects
3.6. Desorption
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morais, S.; Costa, F.G.; Lourdes Pereira, M. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; InTech: London, UK, 2012; pp. 227–244. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury; ATSDR: Atlanta, GA, USA, 1999.
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead; ATSDR: Atlanta, GA, USA, 2007.
- Marín, A.; Gonzalez, V.; Lapo, B.; Molina, E.; Lemus, M. Mercury levels in sediments from the coast of El Oro-Ecuador. Gayana 2016, 80, 147–153. [Google Scholar] [CrossRef]
- Safruk, A.M.; McGregor, E.; Whitfield Aslund, M.L.; Cheung, P.H.; Pinsent, C.; Jackson, B.J.; Hair, A.T.; Lee, M.; Sigal, E.A. The influence of lead content in drinking water, household dust, soil, and paint on blood lead levels of children in Flin Flon, Manitoba and Creighton, Saskatchewan. Sci. Total Environ. 2017, 593–594, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Dwivedi, M.K.; Singh, P.K.; Patel, B.; Pandey, S.; Patel, B.; Patel, A.; Singh, B. Effluences of Heavy Metals, Way of Exposure and Bio-toxic Impacts: An Update. J. Chem. Chem. Sci. 2016, 66, 2319–7625. [Google Scholar]
- Borras Atienzar, C.; Lapo Calderón, B.; Fernandéz Martínez, L. Electroquímica en el Tratamiento de Efluentes (Electrochemical in Wastewater Treatment), 1st ed.; Universidad Técnica de Machala: Machala, Ecuador, 2015; ISBN 978-9978-316-96-2. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Li, N.; Bai, R.; Liu, C. Enhanced and Selective Adsorption of Mercury Ions on Chitosan Beads Grafted with Polyacrylamide via Surface-Initiated Atom Transfer Radical Polymerization. Langmuir 2005, 21, 11780–11787. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, A.; Kahu, S.; Saravanan, D.; Jugade, R. Removal of Cd(II) and Hg(II) from effluents by ionic solid impregnated chitosan. Int. J. Biol. Macromol. 2017, 104, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Kong Yong, S.; Bolan, N.; Lombi, E.; Skinner, W. Enhanced Zn(II) and Pb(II) removal from wastewater using thiolated chitosan beads (ETB). Malays. J. Anal. Sci. 2015, 19, 586–594. [Google Scholar]
- Liu, B.; Chen, W.; Peng, X.; Cao, Q.; Wang, Q.; Wang, D.; Meng, X.; Yu, G. Biosorption of lead from aqueous solutions by ion-imprinted tetraethylenepentamine modified chitosan beads. Int. J. Biol. Macromol. 2016, 86, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Marques Neto, J.D.O.; Bellato, C.R.; Milagres, J.L.; Pessoa, K.D.; Alvarenga, E.S. Preparation and evaluation of chitosan beads immobilized with Iron(III) for the removal of As(III) and As(V) from water. J. Braz. Chem. Soc. 2013, 24, 121–132. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar] [CrossRef]
- Ruiz, M.; Sastre, A.M.; Zikan, M.C.; Guibal, E. Palladium sorption on glutaraldehyde-crosslinked chitosan in fixed-bed systems. J. Appl. Polym. Sci. 2001, 81, 153–165. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A. Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems. Polymers (Basel) 2018, 10, 204. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Virolainen, E.; Conley, K.; Vahala, R. Chitosan-Zinc(II) complexes as a bio-sorbent for the adsorptive abatement of phosphate: Mechanism of complexation and assessment of adsorption performance. Polymers (Basel) 2017, 10, 25. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 5, 385–471. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Phys. Chem. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Valverde Armas, P.; Galarza Romero, B. Caracterización geoquímica e isotópica del agua superficial y subterránea en el área de influencia del río Siete y de las actividades mineras en el distrito minero de Ponce Enriquez, Escuela Superior Politécnica del Litoral. Rev. Derecho Priv. 2013, 62, 465–490. [Google Scholar]
- Vieira, R.S.; Beppu, M.M. Dynamic and static adsorption and desorption of Hg(II) ions on chitosan membranes and spheres. Water Res. 2006, 40, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Sipos, P.; Berkesi, O.; Tombacz, E.; St Pierre, T.G.; Webb, J.; Kalman Burger, P. Formation of spherical iron(III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous solutions. J. Inorg. Biochem. 2003, 95, 55–63. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 967–981. [Google Scholar] [CrossRef]
- Hong, P.-Z.; Li, S.-D.; Ou, C.-Y.; Li, C.-P.; Yang, L.; Zhang, C.-H. Thermogravimetric Analysis of Chitosan. J. Appl. Electrochem. 2007, 449–456. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Huang, Y. Magnetic Chitosan–Iron(III) Hydrogel as a Fast and Reusable Adsorbent for Chromium(VI) Removal. Ind. Eng. Chem. Res. 2013, 52, 11956–11966. [Google Scholar] [CrossRef]
- Lyon, R.E. An integral method of nonisothermal kinetic analysis. Thermochim. Acta 1997, 297, 117–124. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef]
- Jeon, C.; Oll, W.H.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Liu, Z.; Huang, Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M. Swelling-adsorption interactions during mercury and nickel ions removal by chitosan derivatives. Sep. Purif. Technol. 2015, 149, 92–102. [Google Scholar] [CrossRef]
- Shawky, H.A.; El-Aassar, A.H.M.; Abo-Zeid, D.E. Chitosan/carbon nanotube composite beads: Preparation, characterization, and cost evaluation for mercury removal from wastewater of some industrial cities in Egypt. J. Appl. Polym. Sci. 2012, 125, E93–E101. [Google Scholar] [CrossRef]
- Franks, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Colloids Surf. Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420051896. [Google Scholar]
- Krešić, N. Hydrogeology and Groundwater Modeling; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849333484. [Google Scholar]
- Kamari, A.; Wan Saime, W.N.; Lai Ken, L. Chitosan and chemically modified chitosan beads for acid dyes sorption. J. Environ. Sci. 2009, 21, 296–302. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, N.; Tiwari, S.; Tiwari, S.K.; Dhakate, S.R. Cerium functionalized PVA–chitosan composite nanofibers for effective remediation of ultra-low concentrations of Hg(II) in water. RSC Adv. 2015, 5, 16622–16630. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Sastre, A.M.; Guibal, E. Development of a new chitosan/Ni(OH)2-based sorbent for boron removal. Chem. Eng. J. 2014, 244, 576–586. [Google Scholar] [CrossRef]
- Dhanapal, V.; Subramanian, K. Modified chitosan for the collection of reactive blue 4, arsenic and mercury from aqueous media. Carbohydr. Polym. 2015, 117, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, T.; Yan, L.; Guo, X.; Cui, L.; Wei, Q.; Du, B. Preparation of novel cobalt ferrite/chitosan grafted with graphene composite as effective adsorbents for mercury ions. J. Mol. Liq. 2014, 198, 381–387. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, W.; Wang, A. Highly efficient adsorption of Hg(II) and Pb(II) onto chitosan-based granular adsorbent containing thiourea groups. J. Water Process Eng. 2015, 7, 218–226. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Luo, C.; Wang, X.; Duan, H. Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb2+. Int. J. Biol. Macromol. 2016, 86, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Igberase, E.; Osifo, P. Equilibrium, kinetic, thermodynamic and desorption studies of cadmium and lead by polyaniline grafted cross-linked chitosan beads from aqueous solution. J. Ind. Eng. Chem. 2015, 26, 340–347. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin-a biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Modeling competitive sorption of lead and copper ions onto alginate and greenly prepared algal-based beads. Bioresour. Technol. 2017, 231, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Regules-Martínez, M.C.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Carranza-Alvarez, C. Single and competitive adsorption of Cd(II) and Pb(II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste. Sustain. Environ. Res. 2017, 27, 61–69. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Flores-Cano, J.V. Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem. Eng. J. 2013, 225, 536–546. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of Porous-Magnetic Chitosan Beads for Removal of Cadmium Ions from Wastewater. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Kawamura, Y.; Mitsuhashi, M.; Tanibe, H.; Yoshida, H. Adsorption of Metal Ions on Polyaminated Highly Porous Chitosan Chelating Resin. Znd. Eng. Chem. Res. 1993, 32, 386–391. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; Jackson, M.L. Adsorption of Mercury(II) by Iron Hydrous Oxide Gel. Soil Sci. Soc. Am. J. 1978, 42, 45. [Google Scholar] [CrossRef]
- Mitani, T.; Fukumuro, N.; Yoshimoto, C.; Ishii, H.; Inoue, K.; Baba, Y.; Yoshizawa, K.; Noguchi, H.; Yoshizaki, M.; Lett, C. Effects of Counter Ions (Sulfate and Chloride) on the Adsorption of Copper and Nickel Ions by Swollen Chitosan Beads. Chem. Biol. Technol. Agric. 1991, 55, 2419. [Google Scholar]
- Zhu, T. The redox reaction between thiourea and ferric iron and catalysis of sulphide ores. Hydrometallurgy 1992, 28, 381–397. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209–210, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.; Endud, C.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Badruddoza, Z.; Zakir, Z.; Tay, J.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef] [PubMed]










| Single Component System | ||||||
| Hg(II) | Pb(II) | |||||
| Parameter | Unit | Value | Error | Value | Error | |
| Langmuir | qexp | (mmol·g−1) | 1.61 | 0.52 | ||
| qmax | (mmol·g−1) | 1.80 | 0.06 | 0.56 | 0.007 | |
| b | (L·mmol−1) | 10.17 | 1.31 | 38.8 | 2.49 | |
| r2 | 0.988 | 0.996 | ||||
| qmax × b | (L·g−1) | 18.3 | 21.44 | |||
| Freundlich | KF | (mmol1−1/n·g−1·L1/n) | 1.97 | 0.07 | 0.69 | 0.05 |
| n | 2.63 | 0.19 | 3.66 | 0.54 | ||
| r2 | 0.982 | 0.91 | ||||
| Sips | qmax | (mmol·g−1) | 2.3 | 0.26 | 0.55 | 0.01 |
| Ks | (L·mmol−1) | 3.14 | 1.18 | 52.41 | 15.04 | |
| ns | 1.4 | 0.15 | 0.93 | 0.05 | ||
| r2 | 0.995 | 0.998 | ||||
| Binary Component System | ||||||
| Value | Error | |||||
| Langmuir competitive model | Km | (mmol·g−1) | 2.87 | 0.57 | ||
| K1 | (mmol·g−1) | 5.68 | 1.60 | |||
| K2 | (mmol·g−1) | 2.24 | 0.63 | |||
| r2 | 0.96 | |||||
| Modification | Metal | pH | T (°C) | qmax (mmol·M+·g−1) | Isotherm fitting | Ref. |
|---|---|---|---|---|---|---|
| Microspheres chitosan grafted with chlorosulfonic acid (CSSULF) or ethylenimine (CSPEI) | Hg(II) | 6 | - | 0.32 | Langmuir/Freundlich | [35] |
| Cross-linked aminated chitosan beads | Hg(II) | 7 | 2.23 | Langmuir | [33] | |
| Chitosan/Graphene oxide imprinted Pb2+ | Pb(II) | 5 | 30 | 0.38 | Langmuir | [47] |
| Polyaniline grafted cross-linked chitosan beads | Pb(II) | 45 | 0.55 | Langmuir | [48] | |
| Present study: ChiFer(III) | Hg(II) Pb(II) | 4.5 | Room | 1.80 0.56 | Langmuir |
| Experimental | PFORE | PSORE | ||||||
|---|---|---|---|---|---|---|---|---|
| Metal | Sorbent | qexp (mmol·g−1) | K1 (h−1) | q1 (mmol·g−1) | r2 | K2 (g∙mmol−1∙h−1) | q2 (mmol·g−1) | r2 |
| Hg(II) | Air dried beads (AD) | 1.71 | 1.44 | 1.57 | 0.983 | 1.18 | 1.69 | 0.991 |
| Freeze dried beads (FD) | 1.70 | 1.36 | 1.53 | 0.977 | 1.12 | 1.65 | 0.995 | |
| Pb(II) | Freeze dried beads (FD) | 0.64 | 2.09 | 0.63 | 0.930 | 4.94 | 0.679 | 0.945 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. https://doi.org/10.3390/polym10040367
Lapo B, Demey H, Zapata J, Romero C, Sastre AM. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers. 2018; 10(4):367. https://doi.org/10.3390/polym10040367
Chicago/Turabian StyleLapo, Byron, Hary Demey, Jessenia Zapata, Cristhian Romero, and Ana María Sastre. 2018. "Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems" Polymers 10, no. 4: 367. https://doi.org/10.3390/polym10040367
APA StyleLapo, B., Demey, H., Zapata, J., Romero, C., & Sastre, A. M. (2018). Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers, 10(4), 367. https://doi.org/10.3390/polym10040367