Molecular Design of Soluble Biopolyimide with High Rigidity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Syntheses
2.3. Characterization
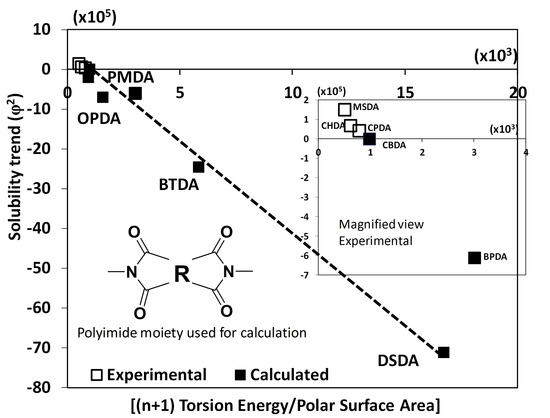
3. Results and Discussion
Biopolyimide Synthesis and Structural Characteristics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, K.L. Polyimides: Synthesis, Characterization and Applications; John Wiley Sons: New York, NY, USA, 1985; Volume 1. [Google Scholar]
- Cassidy, P.E. Thermally Stable Polymers: Synthesis and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Kricheldorf, H.R.; Linzer, V. Liquid crystalline polyimides: 18. Thermotropic polyimides based on biphenyl-3,3′,4,4′-tetracarboxylic anhydride. Polymer 1995, 36, 1893–1902. [Google Scholar] [CrossRef]
- Marek, M.; Doskocilova, D.; Schmidt, P.; Schneider, B.; Kriz, J.; Labsky, J.; Puffer, R. New soluble polyimides prepared from 4,4′-(alkylenediyldioxy)dianilines. Polymer 1994, 35, 4881–4888. [Google Scholar] [CrossRef]
- Acevedo, M.; Harris, F.W. Polyimides derived from 2-methyl-2-propyl-1,3-bis(4-aminophenoxy)propane and 2,2-dimethyl-1,3-bis(4-aminophenoxy)propane. Polymer 1994, 35, 4456–4461. [Google Scholar] [CrossRef]
- Giesa, R.; Keller, U.; Eiselt, P.; Schmidt, H.W. Synthesis and thermal properties of aryl-substituted rod-like polyimides. J. Polym. Sci. A 1993, 31, 141–151. [Google Scholar] [CrossRef]
- Becker, K.H.; Schmidt, H.W. Para-linked aromatic poly(amic ethyl esters): Precursors to rodlike aromatic polyimides. 1. Synthesis and imidization study. Macromolecules 1992, 25, 6784–6790. [Google Scholar] [CrossRef]
- Chung, I.S.; Kim, S.Y. Soluble Polyimides from Unsymmetrical Diamine with Trifluoromethyl Pendent Group. Macromolecules 2000, 33, 3190–3193. [Google Scholar] [CrossRef]
- Takekoshi, T. Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Wei, Q.; Hirota, K.; Tajima, K.; Hashimoto, K. Design and Synthesis of TiO2Nanorod Assemblies and Their Application for Photovoltaic Devices. Chem. Mater. 2006, 18, 5080–5087. [Google Scholar] [CrossRef]
- Kang, H.A.; Chung, I.S.; Kakimoto, M.; Kim, S.Y. Synthesis and Characterization of Polyimides from Unsymmetrical Diamine with Cyano Groups. Polym. J. 2001, 33, 284–289. [Google Scholar] [CrossRef]
- Huang, S.J.; Hoyt, A.E. The synthesis of soluble polyimides. Trends Polym. Sci. 1995, 3, 262–271. [Google Scholar]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Cui, Z.; Lee, J.; Lee, Y.M.; Guiver, M.D. Intrinsically Microporous Soluble Polyimides Incorporating Tröger’s Base for Membrane Gas Separation. Macromolecules 2014, 47, 3254–3262. [Google Scholar] [CrossRef]
- Yagci, H.; Mathias, L. Synthesis and characterization of aromatic polyamides and polyimides from trimethyl- and di-t-butylhydroquinone-based ether-linked diamines. J. Polym. 1998, 39, 3779–3786. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yang, C.P.; Chen, S.H. Synthesis and properties of ortho-linked aromatic polyimides based on 1,2-bis(4-aminophenoxy)-4-tert-butylbenzene. J. Polym. Sci. A 2000, 38, 1551–1559. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y. Synthesis and Properties of Polyimides Derived from 1,4-Bis(4-aminophenoxy)-2-tert-butylbenzene. Polym. J. 1996, 28, 970–975. [Google Scholar] [CrossRef]
- Langsam, M.; Burgoyne, W.F. Effects of diamine monomer structure on the gas permeability of polyimides. I. Bridged diamines. J. Polym. Sci. A 1993, 31, 909–921. [Google Scholar] [CrossRef]
- Huang, W.; Yan, D.; Lu, Q.; Tao, P. Preparation of aromatic polyimides highly soluble in conventional solvents. J. Polym. Sci. A 2002, 40, 229–234. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y.; Li, L.J.; Sillion, B.; Mercier, R.; Thiria, R.; Sekiguchi, H. Synthesis and Characterization of New Soluble Polyimides from 3,3′,4,4′-Benzhydrol Tetracarboxylic Dianhydride and Various Diamines. Chem. Mater. 1998, 10, 734–739. [Google Scholar] [CrossRef]
- Li, F.; Ge, J.J.; Honigfort, P.S.; Fang, S.; Chen, J.C.; Harris, F.W.; Cheng, S.Z.D. Dianhydride architectural effects on the relaxation behaviors and thermal and optical properties of organo-soluble aromatic polyimide films. Polymer 1999, 40, 4987–5002. [Google Scholar] [CrossRef]
- Suvannasara, P.; Tateyama, S.; Miyasato, A.; Matsumura, K.; Shimoda, T.; Ito, T.; Yamagata, Y.; Fujita, T.; Takaya, N.; Kaneko, T. Biobased Polyimides from 4-Aminocinnamic Acid Photodimer. Macromolecules 2014, 47, 1586–1593. [Google Scholar] [CrossRef]
- Tateyama, S.; Masuo, S.; Suvannasara, P.; Oka, Y.; Miyasato, A.; Yasaki, K.; Teerawatananond, T.; Muangsin, N.; Zhou, S.; Kawasaki, Y.; et al. Ultrastrong, Transparent Polytruxillamides Derived from Microbial Photodimers. Macromolecules 2016, 49, 3336–3342. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Herndon, VA, USA, 2006; ISBN 978-1-891389-31-3. [Google Scholar]
- Bicerano, J. Prediction of Polymer Properties; Mercel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Scatchard, G. Equilibria in Non-electrolyte Solutions in Relation to the Vapor Pressures and Densities of the Components. Chem. Rev. 1931, 8, 321–333. [Google Scholar] [CrossRef]
- Hildebrand, J.H. Solubility. J. Am. Chem. Soc. 1916, 38, 1452–1473. [Google Scholar] [CrossRef]
- Hildebrand, J.H. Solubility III Relative values of internal pressures and their practical applications. J. Am. Chem. Soc. 1919, 41, 1067–1080. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Prausnitz, J.M.; Scott, R.L. Regular and Related Solutions: The Solubility of Gases, Liquids, and Solids; Reinhold: New York, NY, USA, 1970. [Google Scholar]
- Chen, X.; Yuan, C.; Wong, C.K.Y.; Zhang, G. Molecular modeling of temperature dependence of solubility parameters for amorphous polymers. J. Mol. Model. 2012, 18, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, N.; Chamjangali, M.A.; Amin, A.H. Calculation of Hildebrand Solubility Parameters of Some Polymers Using QSPR Methods Based on LS-SVM Technique and Theoretical Molecular Descriptors. Chin. J. Polym. Sci. 2014, 32, 587–594. [Google Scholar] [CrossRef]
- Li, L.; Totton, T.; Frenkel, D. Computational methodology for solubility prediction: Application to the sparingly soluble solutes. J. Chem. Phys. 2017, 146, 214110–214115. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; McCabe, G. Introduction to the Practice of Statistics; W. H. Freeman and Co.: London, UK, 2003. [Google Scholar]




| Polyimide | CHDA | MSDA | CPDA |
|---|---|---|---|
| Dianhydride | |||
| Td10 a (°C) | 397 | 375 | 391 |
| Tg b (°C) | 259 | 273 | 294 |
| T c (%) | 51 | 57 | ND |
| Mn d (g/mol) | 4.8 × 105 | 7.8 × 105 | 5.9 × 105 |
| Mw/Mn d | 1.4 | 1.4 | 1.3 |
| Solubility Test | |||
| Hexane | - | - | - |
| Toluene | - | - | - |
| Dichloromethane | - | - | - |
| Chloroform | - | - | - |
| Diethylether | - | - | - |
| Water | - | - | - |
| Methanol | - | - | - |
| Ethanol | - | - | - |
| Acetone | - | - | - |
| Acetonitrile | - | - | - |
| Ethylacetate | - | - | - |
| Tetrahydrofuran | ± | ± | ± |
| N-Methyl-2-pyrrolidone | + | + | + |
| Dimethyl sulfoxide | + | + | + |
| N,N-dimethylformamide | + | + | + |
| N,N-dimethylacetamide | + | + | + |
| Conc. H2SO4 | + | + | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwivedi, S.; Kaneko, T. Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers 2018, 10, 368. https://doi.org/10.3390/polym10040368
Dwivedi S, Kaneko T. Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers. 2018; 10(4):368. https://doi.org/10.3390/polym10040368
Chicago/Turabian StyleDwivedi, Sumant, and Tatsuo Kaneko. 2018. "Molecular Design of Soluble Biopolyimide with High Rigidity" Polymers 10, no. 4: 368. https://doi.org/10.3390/polym10040368
APA StyleDwivedi, S., & Kaneko, T. (2018). Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers, 10(4), 368. https://doi.org/10.3390/polym10040368