High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Methacrylate Oligo-Siloxane (MO) Resins
2.3. Fabrication of Methacrylate Siloxane Hybrid (MSH) Materials
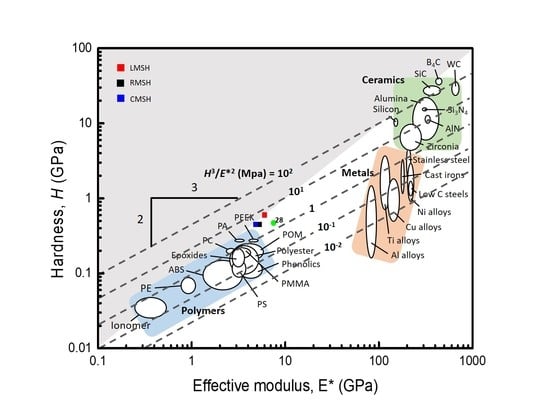
2.4. Characterization
3. Results and Discussion
3.1. Preparation of Methacrylate Oligo-Siloxane Resins
3.2. Molecular Structure Analysis of the MO Resins
3.3. Fabrication of the Methacrylate Siloxane Hybrid Materials (MSH)
3.4. Characterizations of the MSHs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharp, K.G. Inorganic/Organic Hybrid Materials. Adv. Mater. 1998, 10, 1243–1248. [Google Scholar] [CrossRef]
- Novak, B.M. Hybrid Nanocomposite Materials-between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Jin, J.; Lee, J.; Jeong, S.; Yang, S.; Ko, J.-H.; Im, H.-G.; Baek, S.-W.; Lee, J.-Y.; Bae, B.-S. High-performance hybrid plastic films: A robust electrode platform for thin-film optoelectronics. Energy Environ. Sci. 2013, 6, 1811–1817. [Google Scholar] [CrossRef]
- Mammeri, F.; Le Bourhis, E.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Oliver, M.S.; Dubois, G.; Sherwood, M.; Gage, D.M.; Dauskardt, R.H. Molecular Origins of the Mechanical Behavior of Hybrid Glasses. Adv. Funct. Mater. 2010, 20, 2884–2892. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Page, S.J.; Chung, J.J.; Amdursky, N.; Stevens, M.M.; Weaver, J.V.M.; Hanna, J.V.; Jones, J.R. A structural and physical study of sol–gel methacrylate–silica hybrids: Intermolecular spacing dictates the mechanical properties. Phys. Chem. Chem. Phys. 2015, 17, 29124–29133. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.B.; Li, S.; Chung, J.J.; Nommeots-Nomm, A.; Solanki, A.K.; Stevens, M.M.; Jones, J.R. Ductile silica/methacrylate hybrids for bone regeneration. J. Mater. Chem. B 2016, 4, 6032–6042. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, S.; Bae, B.-S. Thermally Stable Transparent Sol–Gel Based Siloxane Hybrid Material with High Refractive Index for Light Emitting Diode (LED) Encapsulation. Chem. Mater. 2010, 22, 3549–3555. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lim, Y.-W.; Kim, Y.H.; Bae, B.-S. Thermally Stable Siloxane Hybrid Matrix with Low Dielectric Loss for Copper-Clad Laminates for High-Frequency Applications. ACS Appl. Mater. Interfaces 2016, 8, 8335–8340. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.-H.; Im, H.-G.; Jang, J.; Jang, D.; Bae, B.-S. Flexible Hard Coating: Glass-Like Wear Resistant, Yet Plastic-Like Compliant, Transparent Protective Coating for Foldable Displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.B.; Kasuga, T.; Remzi Becer, C.; Jones, J.R. Silica/methacrylate class II hybrid: Telomerisation vs. RAFT polymerisation. Polym. Chem. 2017, 8, 3603–3611. [Google Scholar] [CrossRef]
- Jin, J.; Ko, J.-H.; Yang, S.; Bae, B.-S. Rollable Transparent Glass-Fabric Reinforced Composite Substrate for Flexible Devices. Adv. Mater. 2010, 22, 4510–4515. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-Y.; Yang, S.; Jin, J.H.; Jung, K.; Kim, J.-S.; Bae, B.-S. Fabrication of transparent methacrylate zirconium siloxane hybrid materials using sol–gel synthesized oligosiloxane resin. J. Sol-Gel Sci. Technol. 2011, 58, 114–120. [Google Scholar] [CrossRef]
- Bizet, S.; Galy, J.; Gérard, J.-F. Structure–Property Relationships in Organic–Inorganic Nanomaterials Based on Methacryl–POSS and Dimethacrylate Networks. Macromolecules 2006, 39, 2574–2583. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, A.S.; Lee, J.-C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogel electrolytes for high temperature lithium batteries. J. Mater. Chem. A 2015, 3, 2226–2233. [Google Scholar] [CrossRef]
- Zhang, C.; Laine, R.M. Hydrosilylation of Allyl Alcohol with [HSiMe2OSiO1.5]8: Octa(3-hydroxypropyldimethylsiloxy)octasilsesquioxane and Its Octamethacrylate Derivative as Potential Precursors to Hybrid Nanocomposites. J. Am. Chem. Soc. 2000, 122, 6979–6988. [Google Scholar] [CrossRef]
- Choi, J.; Harcup, J.; Yee, A.F.; Zhu, Q.; Laine, R.M. Organic/Inorganic Hybrid Composites from Cubic Silsesquioxanes. J. Am. Chem. Soc. 2001, 123, 11420–11430. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Toyodome, H.; Shoiriki, M.; Iyi, N. Preparation of Ionic Silsesquioxanes with Regular Structures and Their Hybridization. Int. J. Polym. Sci. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, A.S.; Hwang, S.S.; Baek, K.-Y. Structural Control of Fully Condensed Polysilsesquioxanes: Ladderlike vs. Cage Structured Polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]
- Lee, A.S.; Jo, Y.Y.; Choi, S.-S.; Baek, K.-Y.; Hwang, S.S. Thermal, Mechanical, and Photophysical Properties of Carbazole-Substituted POSS and Ladder Polysilsesquioxane. J. Nanosci. Nanotechnol. 2017, 17, 5562–5565. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.S.; Song, S.-J.; Baek, K.-Y.; Hwang, S.S. Cationically photopolymerizable epoxy-functionalized thermoplastic polysilsesquioxanes: Synthesis and properties. RSC Adv. 2014, 4, 56532–56538. [Google Scholar] [CrossRef]
- Lee, A.S.S.; Lee, J.H.; Lee, J.-C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Novel polysilsesquioxane hybrid polymer electrolytes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 1277–1283. [Google Scholar] [CrossRef]
- Shang, X.X.; Duan, S.; Zhang, M.; Cao, X.Y.; Zheng, K.; Zhang, J.N.; Ma, Y.M.; Zhang, R.B. UV-curable ladder-like diphenylsiloxane-bridged methacryl-phenyl-siloxane for high power LED encapsulation. RSC Adv. 2018, 8, 9049–9056. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Sepeur, S.; Kunze, N.; Werner, B.; Schmidt, H. UV curable hard coatings on plastics. Thin Solid Films 1999, 351, 216–219. [Google Scholar] [CrossRef]
- Jin, J.; Yang, S.; Bae, B.-S. Fabrication of a high thermal-stable methacrylate-silicate hybrid nanocomposite: Hydrolytic versus non-hydrolytic sol–gel synthesis of methacryl-oligosiloxanes. J. Sol-Gel Sci. Technol. 2011, 61, 321–327. [Google Scholar] [CrossRef]
- Lopes, G.H.; Junges, J.; Fiorio, R.; Zeni, M.; Zattera, A.J. Thermoplastic polyurethane synthesis using POSS as a chain modifier. Mater. Res. 2012, 15, 698–704. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Hao, J.; Xie, P.; Zhang, X.; Han, C.C.; Zhang, R. A Well-Defined Ladder Polyphenylsilsesquioxane (Ph-LPSQ) Synthesized via a New Three-Step Approach: Monomer Self-Organization–Lyophilization—Surface-Confined Polycondensation. Chem. Mater. 2008, 20, 1322–1330. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, A.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.-Y.; Choi, D.H.; Hwang, S.S. Synthesis and characterization of UV-curable ladder-like polysilsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5012–5018. [Google Scholar] [CrossRef]
- Ishii, T.; Enoki, T.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Preparation of imidazolium-type ionic liquids containing silsesquioxane frameworks and their thermal and ion-conductive properties. RSC Adv. 2015, 5, 15226–15232. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Shi, Y.; Huang, G. Effect of polyhedral oligomeric silsesquioxane (POSS) on crystallization behaviors of POSS/polydimethylsiloxane rubber nanocomposites. RSC Adv. 2014, 4, 6275–6283. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Dickens, S.H. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent. Mater. 2001, 17, 71–79. [Google Scholar] [CrossRef]
- Tyng, L.Y.; Ramli, M.R.; Othman, M.B.H.; Ramli, R.; Ishak, Z.A.M.; Ahmad, Z. Effect of crosslink density on the refractive index of a polysiloxane network based on 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane. Polym. Int. 2013, 62, 382–389. [Google Scholar] [CrossRef]
- Zhang, C.; Guang, S.; Zhu, X.; Xu, H.; Liu, X.; Jiang, M. Mechanism of Dielectric Constant Variation of POSS-Based Organic–Inorganic Molecular Hybrids. J. Phys. Chem. C 2010, 114, 22455–22461. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Musil, J. Flexible hard nanocomposite coatings. RSC Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Musil, J.; Blazek, J.; Rajfrlik, K.; Cerstvy, R. Flexible antibacterial Al–Cu–N films. Surf. Coat. Technol. 2015, 264, 114–120. [Google Scholar] [CrossRef]
- Zok, F.W.; Miserez, A. Property maps for abrasion resistance of materials. Acta Mater. 2007, 55, 6365–6371. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.Y.; Pharr, G.M.; Oliver, W.C.; Bhatia, C.S.; White, R.L.; Anders, S.; Anders, A.; Brown, I.G. Nanoindentation and Nanoscratching of Hard Carbon Coatings for Magnetic Disks. MRS Proc. 1995, 383, 447. [Google Scholar] [CrossRef]





| Sample | Methacrylate Conversion | Refractive Index at 633 nm | Transmittance at 550 nm | 5 wt % Decomposition Temperature, T5 wt % | Hardness, H | Effective Modulus, E* | H/E* | Elastic Recovery, We |
|---|---|---|---|---|---|---|---|---|
| % | % | °C | GPa | GPa | % | |||
| LMSH | 71.8 | 1.5043 | 90.6 | 403.77 | 0.6 | 6 | 0.1 | 86.1 |
| RMSH | 77.8 | 1.5032 | 91.3 | 384.76 | 0.45 | 5.29 | 0.085 | 79.5 |
| CMSH | 66.8 | 1.4988 | 91.1 | 393.83 | 0.45 | 4.85 | 0.093 | 83.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Choi, G.-M.; Bae, J.G.; Kim, Y.H.; Bae, B.-S. High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating. Polymers 2018, 10, 449. https://doi.org/10.3390/polym10040449
Kim YH, Choi G-M, Bae JG, Kim YH, Bae B-S. High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating. Polymers. 2018; 10(4):449. https://doi.org/10.3390/polym10040449
Chicago/Turabian StyleKim, Yun Hyeok, Gwang-Mun Choi, Jin Gyu Bae, Yong Ho Kim, and Byeong-Soo Bae. 2018. "High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating" Polymers 10, no. 4: 449. https://doi.org/10.3390/polym10040449
APA StyleKim, Y. H., Choi, G. -M., Bae, J. G., Kim, Y. H., & Bae, B. -S. (2018). High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating. Polymers, 10(4), 449. https://doi.org/10.3390/polym10040449