Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro
Abstract
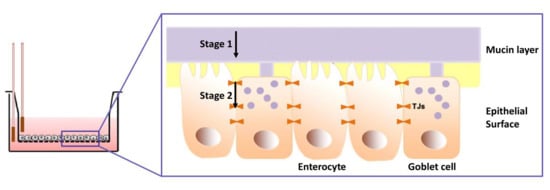
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthetic Methods
2.2.1. Synthesis of CS-g-PMMA Copolymer
2.2.2. Synthesis of Thiolated CS-g-PMMA Copolymer
2.3. Characterization Methods
2.3.1. Proton Nuclear Magnetic Resonance Spectroscopy
2.3.2. Fourier Transform Infrared Spectroscopy
2.3.3. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
2.3.4. Determination of NAC Content
2.3.5. Self-Assembly
2.3.6. Preparation of Crosslinked CS-g-PMMA Nanoparticles
2.3.7. Size, Size Distribution, and Zeta-Potential
2.3.8. Cell Compatibility of the Copolymers In Vitro
Caco-2 Cell Line
HT29-MTX Cell Line
Cell Compatibility in a Co-Culture of Caco-2 and HT29-MTX Cell Lines
2.3.9. Mucin Staining
2.3.10. Permeability Studies
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of CS-g-PMMA Copolymer
3.2. Synthesis and Chemical Charcterization of Thiolated CS-g-PMMA Copolymer
3.3. Self-Assembly
3.4. Cell Compatibility of the Copolymers In Vitro
3.5. Mucin Staining
3.6. Permeability Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavelle, E.C.; Sharif, S.; Thomas, N.W.; Holland, J.; Davis, S.S. The importance of gastrointestinal uptake of particles in the design of oral delivery systems. Adv. Drug Deliv. Rev. 1995, 18, 5–22. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lai, S.K.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Rossen, J.W.A.; Büller, H.A.; Einerhand, A.W.C. The MUC family: An obituary. Trends Biochem. Sci. 2002, 27, 126–131. [Google Scholar] [CrossRef]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Araújo, F.; Sarmento, B.; Hirvonen, J.; Santos, H.A. Cell-based in vitro models for buccal permeability studies. In Concepts Models for Drug Permeability Studies; Cell Tissue Based In Vitro Culture Models; Elsevier: New York, NY, USA, 2015; pp. 31–40. [Google Scholar] [CrossRef]
- Nunes, R.; Silva, C.; Chaves, L. 4.-Tissue-based in vitro and ex vivo models for intestinal permeability studies A2- Sarmento, Bruno. In Concepts Models for Drug Permeability Studies; Elsevier: New York, NY, USA, 2016; pp. 203–236. [Google Scholar]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Kim, K.-J. Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Springer: Berlin, Germany, 2007. [Google Scholar] [CrossRef]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Janich, S.; Roessler, B.J.; Hilfinger, J.M.; Amidon, G.L. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro-in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996, 85, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Grabovac, V.; Palmberger, T.F.; Hoffer, M.H.; Bernkop-Schnürch, A. Synthesis and characterization of a chitosan-N-acetyl cysteine conjugate. Int. J. Pharm. 2008, 347, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Andrews, G.P.; Jones, D.S. Mucoadhesion and characterization of mucoadhesive properties, In Mucosal Delivery of Biopharmaceuticals Biology Challenges Strategy; Springer: Boston, MA, USA, 2014; pp. 35–58. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Neves, J.D.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Kast, C.E.; Bernkop-Schnürch, A. Thiolated polymers-thiomers: Development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Greimel, A. Thiomers: The next generation of mucoadhesive polymers. Am. J. Drug Deliv. 2005, 3, 141–154. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Sosnik, A.; Raskin, M.M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Raskin, M.M.; Schlachet, I.; Sosnik, A. Mucoadhesive nanogels by ionotropic crosslinking of chitosan-g-oligo(NiPAam) polymeric micelles as novel drug nanocarriers. Nanomedicine (Lond.) 2016, 11, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery, 1st ed.; Elsevier Inc.: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Schlachet, I.; Sosnik, A. Protoporphyrin IX-modified chitosan-g-oligo(NiPAam) polymeric micelles: From physical stabilization to permeability characterization in vitro. Biomater. Sci. 2017, 5, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, B.; Kim, K.S.; Bae, W.J.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Kim, S.W. The potential role of polymethyl methacrylate as a new packaging material for the implantable medical device in the bladder. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Benini, A. PMMA as Drug Delivery System and in vivo Release from Spacers. In Infection and Local Treatment in Orthopedic Surgery; Meani, E., Romanò, C., Crosby, L., Hofmann, G., Calonego, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 79–91. [Google Scholar]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly(Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Chang, T.; Gosain, P.; Stenzel, M.H.; Lord, M.S. Drug-loading of poly(ethylene glycol methyl ether methacrylate) (PEGMEMA)-based micelles and mechanisms of uptake in colon carcinoma cells. Colloids Surf. B Biointerfaces 2016, 144, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Subramanian, K.G.; Vijayakumar, V. Synthesis and evaluation of chitosan-graft-poly(2-hydroxyethyl methacrylate-co-itaconic acid) as a drug carrier for controlled release of tramadol hydrochloride. Saudi Pharm. J. 2012, 20, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Salecan-Based pH-Sensitive Hydrogels for Insulin Delivery. Mol. Pharm. 2017, 14, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Borgström, L.; Kägedal, B.; Paulsen, O. Pharmacokinetics of N-Acetylcysteine in Man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Lai, S.K.; Boylan, N.J.; Dawson, M.R.; Boyle, M.P.; Hanes, J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine (Lond.) 2011, 6, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.C.; Stramiello, L.M.S. Polymer bound EDC (P-EDC): A convenient reagent for formation of an amide bond. Tetrahedron Lett. 1993, 34, 7685–7688. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection. Air Pollution in Haifa Bay; Ministry of Environmental Protection: Beijing, China, 2015; pp. 17–21. Available online: http://www.sviva.gov.il/English/env_topics/IndustryAndBusinessLicensing/Haifa-Bay-Industrial-Zone/Pages/Air-Pollution-in-Haifa-Bay.aspx (accessed on 18 April 2018).
- Hombach, J.; Hoyer, H.; Bernkop-Schnürch, A. Thiolated chitosans: Development and in vitro evaluation of an oral tobramycin sulphate delivery system. Eur. J. Pharm. Sci. 2008, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 10993-5, Biological Evaluation of Medical Devices, 3rd ed.; Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Loh, J.W.; Saunders, M.; Lim, L.Y. Cytotoxicity of monodispersed chitosan nanoparticles against the Caco-2 cells. Toxicol. Appl. Pharmacol. 2012, 262, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan-caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohydr. Polym. 2012, 89, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Loretz, B.; Bernkop-Schnürch, A. In vitro cytotoxicity testing of non-thiolated and thiolated chitosan nanoparticles for oral gene delivery. Nanotoxicology 2009, 1, 139–148. [Google Scholar] [CrossRef]
- Kameyama, A.; Dong, W.; Matsuno, Y.K. Succinylation-Alcian blue staining of mucins on polyvinylidene difluoride membranes. Methods Mol. Biol. 2015, 1314, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.F.; Cristea, M.; Yang, Y.; Winnik, F.M. Engineering polysaccharide-based polymeric micelles to enhance permeability of cyclosporin A across Caco-2 cells. Pharm. Res. 2005, 22, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.M.; Reynolds, F.; Merkle, H.P.; Weissleder, R.; Josephson, L. Transport of surface-modified nanoparticles through cell monolayers. ChemBioChem 2005, 6, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine, Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H. Permeability Methods. Drug-Like Prop. 2016, 325–337. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796. [Google Scholar] [CrossRef] [PubMed]









| Sample | S/SH area ratio |
|---|---|
| NAC | 3.5 |
| Unmodified CS-g-PMMA | 10.8 |
| Thiolated CS-g-PMMA | 3.9 |
| Copolymer | CMC (% w/v) | |
|---|---|---|
| 25 °C | 37 °C | |
| Unmodified CS-g-PMMA | 0.05 | 0.05 |
| Thiolated CS-g-PMMA | 0.05 | 0.04 |
| Copolymer | T [°C] | Non-crosslinked nanoparticles | Crosslinked nanoparticles | ||||
|---|---|---|---|---|---|---|---|
| Dh (nm) ± S.D. (Relative intensity %) | PDI | Z-Potential (mV) | Dh (nm) ± S.D. (Relative intensity %) | PDI | Z-Potential (mV) | ||
| Unmodified CS-g-PMMA | 25 | 127 ± 9 (100) | 0.389 | +27 | 241 ± 11 (100) | 0.192 | +18 |
| Thiolated CS-g-PMMA | 156 ± 6 (94) 25 ± 3 (6) | 0.288 | +33 | 221 ± 11 (100) | 0.237 | +26 | |
| Unmodified CS-g-PMMA | 37 | 184 ± 4 (100) | 0.201 | +25 | 332 ± 52 (100) | 0.336 | +17 |
| Thiolated CS-g-PMMA | 155 ± 5 (94) 28 ± 1 (6) | 0.282 | +37 | 192 ± 5 (100) | 0.228 | +29 | |
| Sample | Crosslinking | Concentration (% w/v) | Papp ± S.D. (10−6 cm/s) |
|---|---|---|---|
| Caco-2 monolayer | |||
| Unmodified CS-g-PMMA | Yes | 0.01 | 2.997 ± 0.455 |
| No | 0.05 | 2.087 ± 0.226 | |
| Yes | 0.05 | 1.215 ± 0.245 | |
| Thiolated CS-g-PMMA | Yes | 0.01 | 3.498 ± 0.682 |
| No | 0.05 | 2.591 ± 0.160 | |
| Yes | 0.05 | 0.921 ± 0.399 | |
| Co-Culture monolayer | |||
| Unmodified CS-g-PMMA | Yes | 0.01 | 3.660 ± 0.915 |
| No | 0.05 | 2.060 ± 0.147 | |
| Yes | 0.05 | 1.462 ± 0.243 | |
| Thiolated CS-g-PMMA | Yes | 0.01 | 2.125 ± 0.460 |
| No | 0.05 | 0.713 ± 0.251 | |
| Yes | 0.05 | 0.912 ± 0.150 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noi, I.; Schlachet, I.; Kumarasamy, M.; Sosnik, A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers 2018, 10, 478. https://doi.org/10.3390/polym10050478
Noi I, Schlachet I, Kumarasamy M, Sosnik A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers. 2018; 10(5):478. https://doi.org/10.3390/polym10050478
Chicago/Turabian StyleNoi, Imrit, Inbar Schlachet, Murali Kumarasamy, and Alejandro Sosnik. 2018. "Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro" Polymers 10, no. 5: 478. https://doi.org/10.3390/polym10050478
APA StyleNoi, I., Schlachet, I., Kumarasamy, M., & Sosnik, A. (2018). Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers, 10(5), 478. https://doi.org/10.3390/polym10050478