Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings
Abstract
:1. Introduction
2. Materials and Methods
- Experiment 1-Exposure of laccase solution in water to plasma. A solution of laccase in water covering a glass slide was subjected to pure Helium Corona pin-to-plane jet plasma. The experimental factors were the applied voltage used for plasma generation and the time of the exposure of the solution to plasma. The experimental response was the laccase activity % relative to the non-plasma treated control solution.
- Experiment 2-Plasma deposition of laccase onto water. A laccase solution was injected into a Corona plasma jet directed onto a film of water lying on top of a glass slide. The experimental factors were, again, the applied voltage and the time of exposure to plasma. The response was laccase activity %.
- Experiment 3-Plasma deposition of solid laccase coatings onto glass slides–optimization of plasma generation voltage. Laccase solution was injected into a Corona plasma jet directed onto a glass slide. The experimental factor was the applied voltage. The response was laccase activity nkat/L.
- Experiment 4-Plasma deposition of solid laccase coatings onto glass slides—optimization of Helium carrier gas flow rate. Done as per Experiment No. 3. The experimental factor was the flow rate of Helium carrier gas and the response was laccase activity nkat/L.
2.1. Preparation of Solid Support or Matrix
2.2. Laccase Source and its Purification
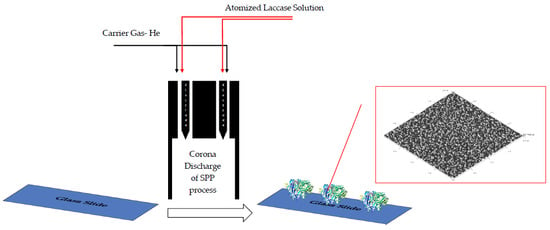
2.3. Laccase Dispensing into the Corona SPP System and Coating
2.4. Water Wash
2.5. Laccase Activity Determination
2.6. Polymerized Particle Size and Zeta Potential
2.7. EDX Composition and AFM Micrographs
2.8. Molecular Weight of Laccase Preparation Proteins and Their Profile
2.9. Fourier Transform Infrared Spectroscopy (FTIR) Measurement
2.10. Long-Term Stability of Laccase Biocoatings
2.11. Circular Dichroism (CD) Spectroscopy of Laccase
3. Results & Discussions
3.1. Experiment 1-Exposure of Laccase Solution in Water to Plasma
3.2. Experiment 2-Plasma Deposition of Laccase onto Water
3.3. Experiment 3-Plasma Deposition of Solid Laccase Coatings onto Glass Slides—Optimization of Plasma Generation Voltage
3.4. Experiment 4-Plasma Deposition of Solid Laccase Coatings onto Glass Slides—Optimization of Helium Carrier Gas Flow Rate
3.5. Durability of Corona SPP Deposited Laccase Biocoating
3.6. Study of Plasma Polymerization Reactions
3.6.1. Molecular Weight Distribution
3.6.2. Particle Size Distribution
3.6.3. Zeta Potential
3.6.4. Circular Dichroism
3.7. Structural and Composition Studies of Coatings
3.7.1. Energy Dispersive X-ray Analysis
3.7.2. Atomic Force Microscopy
3.7.3. Fourier Transform Infra-Red Spectroscopy
3.8. Mechanisms of Corona SPP Laccase Biocoating Deposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van denbosshe, M.; Bernard, L.; Rupper, P.; Maniura-Weber, K.; Heuberger, M.; Faccio, G.; Hegemnn, D. Micro-patterned plasma polymer films for bio-sensing. Mater. Des. 2017, 114, 123–128. [Google Scholar] [CrossRef]
- Herbert, P.A.F.; O’Neill, L.; Jaroszyńska-Wolińska, J. Soft plasma polymerization of gas state precursors from an atmospheric pressure corona plasma discharge. Chem. Mater. 2009, 21, 4401–4407. [Google Scholar] [CrossRef]
- Guimond, S.; Schutz, U.; Hanselmann, B.; Kornr, E.; Hegemann, D. Densification of functional plasma polymers by momentum transfer during film growth. Surf. Coat. Technol. 2011, 205, S447–S450. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma Polymerization—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Nowling, G.; Babayan, S.; Yang, X.; Moravej, M.; Yajima, M.; Hicks, R.; Hoffman, W. Atmospheric plasma deposition of abrasion resistant coatings on plastic. In Proceedings of the IEEE Conference Record, Baltimore, MD, USA, 28 June–1 July 2004. [Google Scholar]
- Nwakira, C.E.; Favaro, G.; Duong, Q.H.; Dowling, D.P. Enhancing the mechanical properties of superhydrophobic atmospheric pressure plasma deposited siloxane coatings. Plasma Process. Polym. 2011, 8, 305–315. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, C.H.; Su, C.H.; Chen, H. Plasma technologies for textile and apparel. Thin Solid Films 2009, 517, 5284–5287. [Google Scholar] [CrossRef]
- Deng, X.; Nikiforow, A.Y.; De Geyter, N.; Morent, R. Deposition of a TMDSO-Based film by a non-equilibrium atmospheric pressure dc plasma jet. Plasma Process. Polym. 2013, 10, 641–648. [Google Scholar] [CrossRef]
- Ward, L.J.; Schofield, C.E.; Badyal, J.P.S. Atmospheric pressure plasma deposition of structurally well-defined polyacrylic acid films. Chem. Mater. 2003, 15, 1466–1469. [Google Scholar] [CrossRef]
- Von Keudell, A.; Awakowicz, P.; Benedict, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Flotgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Process. Polym. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Coad, B.R.; Jasieniak, M.; Griesse, S.S.; Griesser, H.J. Controlled covalent surface immobilisation of proteins and peptides using plasma methods. Surf. Coat. Technol. 2013, 233, 169–177. [Google Scholar] [CrossRef]
- Elagi, A.; Belhacene, K.; Vivien, C.; Dhulster, P.; Froidevaux, R.; Supiot, P. Facile immobilization of enzyme by entrapment using a plasma-deposited organosilicon thin film. J. Mol. Catal. B Enzym. 2014, 110, 77–86. [Google Scholar] [CrossRef]
- Truica-Marasescu, F.; Wertheimer, M.R. Nitrogen-rich plasma-polymer films for biomedical applications. Plasma Process. Polym. 2008, 5, 44–57. [Google Scholar] [CrossRef]
- Mason, M.; Vercruysse, K.P.; Kirker, K.R.; Frish, R.; Marecak, D.M.; Prestwish, G.D.; Pitt, W.G. Attachment of hyaluronic acid to polypropylene, polystyrene, and polytetrafluoroethylene. Biomaterials 2000, 21, 31–36. [Google Scholar] [CrossRef]
- Thyen, R.; Weber, A.; Klages, C.P. Plasma-Enhanced chemical vapor deposition of Thin-Films by corona discharge at atmospheric pressure. Surf. Coat. Technol. 1997, 97, 426–434. [Google Scholar] [CrossRef]
- Gancarz, I.; Bryjak, J.; Poźniak, G.; Tylus, W. Plasma modified polymers as a support for enzyme immobilization 1.: Allyl alcohol plasma. Eur. Polym. J. 2003, 39, 2217–2224. [Google Scholar] [CrossRef]
- Oktem, H.A.; Senyurt, O.; Eyidogan, F.I.; Bayrac, C.; Yilmaz, R. Development of a laccase based paper biosensor for the detection of phenolic compounds. J. Food Agric. Environ. 2012, 10, 1030–1034. [Google Scholar] [CrossRef]
- Fὅrch, R.; Zhang, Z.; Knoll, W. Soft plasma treated Surfaces: Tailoring of structure and properties for biomaterial applications. Plasma Process. Polym. 2005, 2, 351–372. [Google Scholar] [CrossRef]
- Guo, B.; Li, S.; Song, L.; Yang, M.; Zhou, W.; Tyagi, D.; Zhu, J. Plasma-treated polystyrene film that enhances binding efficiency for sensitive and label-free protein biosensing. Appl. Surf. Sci. 2015, 345, 379–386. [Google Scholar] [CrossRef]
- Heyse, P.; Van Hoeck, A.; Roeffaers, M.B.J.; Raffin, J.P.; Steinbuchel, A.; Stoveken, T.; Lammertyn, J.; Verboven, P.; Jacobs, P.A.; Hofkens, J.; et al. Exploration of atmospheric pressure plasma nanofilm technology for straightforward bio-active coating deposition: Enzymes, plasmas and polymers, an elegant synergy. Plasma Process. Polym. 2011, 8, 965–974. [Google Scholar] [CrossRef]
- Palumbo, F.; Camporeale, G.; Yang, Y.W.; Wu, J.S.; Sardella, E.; Dilecce, G.; Calvano, C.D.; Quintieri, L.; Caputo, L.; Baruzzi, F.; et al. Direct plasma deposition of lysozyme-embedded bio-composite thin Films. Plama Process. Polym. 2015, 12, 1302–1310. [Google Scholar] [CrossRef]
- Sheldon, R.A. sheldona, enzyme Immobilization: The quest for optimum performance. Adv. Catal. Synth. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Leonowicz, A.; Rogalski, J.; Jaszek, M.; Luterek, J.; Wojtas-Wasilewska, M.; Malarczyk, E.; Ginalska, G.; Fink-Boots, M.; Cho, N.S. Cooperation of fungal laccase and glucose 1-oxidase in transformation of björkman lignin and some phenolic compounds. Holzforschung 1999, 53, 376–380. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Rogalski, J.; Leonowicz, A. Encyclopedia on Bioresource Technology; Haworth Press: Philadelphia, PA, USA, 2004. [Google Scholar]
- Mata, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Michota, A.; Bukowska, J.; Shleev, S.; Gorton, L.; Bilewicz, R. Properties of native and hydrophobic laccases immobilized in the liquid-crystalline cubic phase on electrodes. J. Biol. Inorg. Chem. 2007, 12, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Lisova, Z.A.; Lisov, A.V.; Leontievsky, A.A. Two laccase isoforms of the basidiomycete Cerrena unicolor VKMF-3196. Induction, isolation and properties. J. Basic Microb. 2010, 50, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Matharu, Z.; Bandodkar, A.J.; Gupta, V.; Malhotra, B.D. Fundamentals and application of ordered molecular assemblies to affinity biosensing. Chem. Soc. Rev. 2012, 41, 1363–1402. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Lee, Y.J. Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch. Biochem. Biophys. 2000, 384, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.rcsb.org/pdb/explore/jmol.do?structureId=1GYC&bionumber=1 (accessed on 13 May 2018).
- Available online: https://www.rcsb.org/pdb/explore/explore.do?structureId=1v10 (accessed on 13 May 2018).
- Rola, B.; Karaśkiewicz, M.; Majdecka, D.; Mazur, J.; Bilewicz, R.; Ohga, S.; Rogalski, J. The large scale Production of cerrena unicolor laccase on waste agricultural based media. J. Agric. Fac. Kyushu Univ. 2015, 60, 297–302. [Google Scholar]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as substrate. Enz. Microbiol. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Agilent High Sensitivity Protein 250 Kit Guide, Agilent Technologies Manual, Reference Number G2938-90310; Agilent Technologies: Waldbronn, Germany, 2016.
- Ahnert, N.; Dalling, J.; Goodman, T.; Thomas, G.A.; Beaudet, M. P146-M A Fast, Easy, Accurate Method for Protein Quantitation. J. Biomol. Tech. 2007, 18, 51. [Google Scholar]
- Ardhaoui, M.; Bhatt, S.; Zheng, M.; Dowling, D.; Jolivalt, C.; Khonsari, F.A. Biosensor based on laccase immobilized on plasma polymerized allylamine/carbon electrode. Mater. Sci. Eng. C 2013, 33, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Huang, K.; Wang, J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control 2015, 50, 482–490. [Google Scholar] [CrossRef]
- Rogalski, J.; Janusz, G. Purification of extracellular laccase from Cerrena unicolor. Biochem. Biotechnol. 2010, 40, 242–255. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Janusz, G.; Kaczmarczyk, I.; Rogalski, J. Chemical modifications of laccase from white-rot basidiomycete Cerrena unicolor. Appl. Biochem. Biotechnol. 2012, 168, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the effects of high hydrostatic pressure in biotechnological applications. Proteins 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, J.J.; Innocente, N. Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase. Food Bioprod. Process. 2016, 98, 181–188. [Google Scholar] [CrossRef]
- Attri, P.; Kim, M.; Sarinont, T.; Choi, E.H.; Seo, H.; Cho, A.E.; Koga, K.; Shiratani, M. Influence of ionic liquid and ionic salt on protein against the reactive species generated using dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 8698–8711. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Fabbro, A.; Federica Buchini, F.; Lippe, G.; Cullen, P.J.; Innocente, N. Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Res. Int. 2014, 66, 365–372. [Google Scholar] [CrossRef]
- Yao, C.W.; Sebastian, D.; Lian, I.; Gunaydin-Sen, O.; Clarke, R.; Clayton, K.; Chem, C.Y.; Kharel, K.; Chen, Y.; Li, Q. Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution. Coatings 2018, 8, 70. [Google Scholar] [CrossRef]
- Takaj, E.; Kitano, K.; Kuwabara, J.; Shiraki, K. Protein Inactivation by Low-temperature Atmospheric Pressure Plasma in Aqueous Solution. Plasma Process. Polym. 2012, 9, 77–82. [Google Scholar] [CrossRef]
- El-Batal, A.; Yassin, A.S.; Amin, M. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sovilij, S.P.; Vickovic, G.; Leovec, V.M.; Minić, D.M. Dinucleas Copper(II) Complexes of N,N′,N″,N‴-Tetrakis(2-pyridylmethyl)-1,4,8,11-tetraazacyclotetradecane and Some N,S or N,O Bidentate Ligands. Pol. J. Chem. 2000, 74, 945–954. [Google Scholar]












| Percentage Content [%] | ||
|---|---|---|
| Non-Plasma Treated Laccase | Plasma Treated Laccase (U = 8 kV, VHe = 10 L/min, t = 60 s) | |
| Helix | 6.3 | 12.7 |
| Antiparallel | 47.9 | 32.2 |
| Parallel | 3.7 | 4.5 |
| Beta-turn | 16.8 | 20.3 |
| Rndm. Coil | 29.3 | 32.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowski, S.; Herbert, P.A.F.; Rogalski, J.; Jaroszyńska-Wolińska, J. Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings. Polymers 2018, 10, 532. https://doi.org/10.3390/polym10050532
Malinowski S, Herbert PAF, Rogalski J, Jaroszyńska-Wolińska J. Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings. Polymers. 2018; 10(5):532. https://doi.org/10.3390/polym10050532
Chicago/Turabian StyleMalinowski, Szymon, P. Anthony F. Herbert, Jerzy Rogalski, and Justyna Jaroszyńska-Wolińska. 2018. "Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings" Polymers 10, no. 5: 532. https://doi.org/10.3390/polym10050532
APA StyleMalinowski, S., Herbert, P. A. F., Rogalski, J., & Jaroszyńska-Wolińska, J. (2018). Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings. Polymers, 10(5), 532. https://doi.org/10.3390/polym10050532