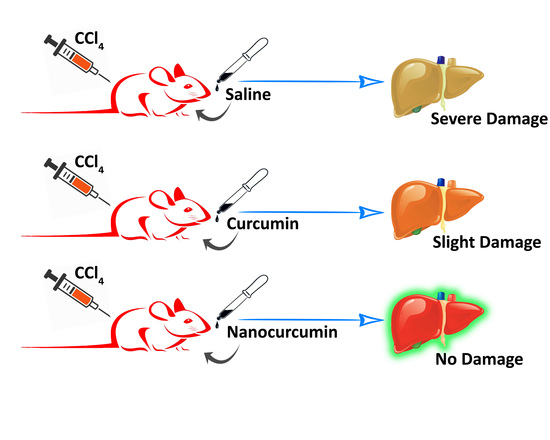
Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl4-Induced Subacute Hepatotoxicity in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Curcumin Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Encapsulation Efficiency
2.5. In vitro drug release
2.6. Experimental Animals
2.7. Experimental Setup
2.8. Biochemical and Histopathological Analysis of Liver
2.9. Statistical Analyses of Data
3. Results
3.1. Characteristic Features of Prepared Nanoparticles
3.2. Drug Release Profile
3.3. Behavioral Changes
3.4. Changes in Liver Damage Marker Enzymes
3.5. Histopathology of the Liver
4. Discussion
5. Conclusions
Author Contribution
Acknowledgments
Conflicts of Interest
References
- Kandimalla, R.; Kalita, S.; Saikia, B.; Choudhury, B.; Singh, Y.P.; Kalita, K.; Dash, S.; Kotoky, J. Antioxidant and hepatoprotective potentiality of Randia dumetorum Lam. Leaf and bark via inhibition of oxidative stress and inflammatory cytokines. Front. Pharmacol. 2016, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.A.; Murthy, P.B.; Pillai, K.S. Screening of hepatoprotective effect of a herbal mixture against CCl4 induced hepatotoxicity in swiss albino mice. J. Environ. Biol. 2007, 28, 201–207. [Google Scholar] [PubMed]
- Marslin, G.; Sarmento, B.F.; Franklin, G.; Martins, J.A.; Silva, C.J.; Gomes, A.F.; Sarria, M.P.; Coutinho, O.M.; Dias, A.C. Curcumin encapsulated into methoxy poly(ethylene glycol) poly(ε-caprolactone) nanoparticles increases cellular uptake and neuroprotective effect in glioma cells. Planta Med. 2017, 83, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.E.; Pearson, M.; Weiner, S.A.; Rajendran, V.; Rubin, D.; Glockner-Pagel, J.; Canny, S.; Du, K.; Lukacs, G.L.; Caplan, M.J. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004, 304, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwitchawan, T.; Chaicharoenaudomrung, N.; Namkaew, J.; Noisa, P. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy. Neurosci. Lett. 2017, 636, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.S.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zhao, Y.; Bao, W.; Xiao, X.; Wang, D.; Nussler, A.K.; Yan, H.; Yao, P.; Liu, L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ros generation and enhancing antioxidative capacity. Phytomedicine 2012, 19, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Z.; Li, H.; Guo, M.; Yang, T.; Feng, S.; Xu, B.; Deng, Y. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and nrf2-are pathway activation. Hum. Exp. Toxicol. 2017, 36, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, X.; Wang, J.; He, X.; Hu, Y.; Zhang, P.; Wang, R.; Li, R.; Gong, M.; Luo, S. Curcumin protects against CCl4-induced liver fibrosis in rats by inhibiting hif-1α through an erk-dependent pathway. Molecules 2014, 19, 18767–18780. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Lin, Y.; Li, X.; Shen, X.; Wang, J.; Tu, C. Curcumin ameliorates intrahepatic angiogenesis and capillarization of the sinusoids in carbon tetrachloride-induced rat liver fibrosis. Toxicol. Lett. 2013, 222, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Ponrasu, T.; Subamekala, M.K.; Janani, M.; Suguna, L. Curcumin loaded on pullulan acetate nanoparticles protects the liver from damage induced by DEN. RSC Adv. 2016, 6, 5599–5610. [Google Scholar] [CrossRef]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin nanoformulation for cervical cancer treatment. Sci. Rep. 2016, 6, 20051. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ge, X.; Wang, L. Construction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin: A synergistic combination nanotherapy for cervical cancer. Biomed. Pharmacother. 2017, 86, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in alzheimer’s disease model via canonical wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Loch-Neckel, G.; Santos-Bubniak, L.; Mazzarino, L.; Jacques, A.V.; Moccelin, B.; Santos-Silva, M.C.; Lemos-Senna, E. Orally administered chitosan-coated polycaprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J. Pharm. Sci. 2015, 104, 3524–3534. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Khan, M.A.; Bekhit, M.; Bai, H.; Cornish, T.; Mizuma, M.; Rudek, M.A.; Zhao, M.; Maitra, A.; Ray, B.; et al. A polymeric nanoparticle formulation of curcumin (nanocurc™) am eliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab. Invest. 2011, 91, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.T.; Das, N.; Ghosh, S.; Ghosh, D.; Chakraborty, S.; Ali, N. Vesicular (liposomal and nanoparticulated) delivery of curcumin: A comparative study on carbon tetrachloride–mediated oxidative hepatocellular damage in rat model. Int. J. Nanomed. 2016, 11, 2179–2193. [Google Scholar]
- Shah, M.K.; Madan, P.; Lin, S. Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration. Pharm. Dev. Technol. 2014, 19, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Cheng, K.-M.; Huang, H.-Y.; Chao, P.-Y.; Hwang, J.-M.; Lee, H.-H.; Lu, C.-Y.; Chiu, Y.-W.; Liu, J.-Y. Hepatoprotective activity of chhit-chan-than extract powder against carbon tetrachloride-induced liver injury in rats. J. Food Drug Anal. 2014, 22, 220–229. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Kalaichelvan, V.K.; Manavalan, R.; Reddy, P.N.; Franklin, G. Poly(d,l-lactic-co-glycolic acid) nanoencapsulation reduces erlotinib-induced subacute toxicity in rat. J. Biomed. Nanotechnol. 2009, 5, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Revina, A.M.; Khandelwal, V.K.; Balakumar, K.; Sheeba, C.J.; Franklin, G. PEGylated ofloxacin nanoparticles render strong antibacterial activity against many clinically important human pathogens. Colloids Surf. B Biointerfaces 2015, 132, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Revina, A.M.; Khandelwal, V.K.; Balakumar, K.; Prakash, J.; Franklin, G.; Sheeba, C.J. Delivery as nanoparticles reduces imatinib mesylate-induced cardiotoxicity and improves anticancer activity. Int. J. Nanomed. 2015, 10, 3163–3170. [Google Scholar]
- Torres, L.R.; Santana, F.C.; Torres-Leal, F.L.; Melo, I.L.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.; Araujo, C.M.; Cogliati, B.; Mancini-Filho, J. Pequi (caryocar brasiliense camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, F.; Tang, J.; Mao, A.; Liao, S.; Wang, Q. Dicranostiga leptopodu (maxim.) fedde extracts attenuated CCl4-induced acute liver damage in mice through increasing anti-oxidative enzyme activity to improve mitochondrial function. Biomed. Pharmacother. 2017, 85, 763–771. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; Lebda, M.A.; Hassinin, M.; Neoman, S.A. Chicory (Cichorium intybus l.) root extract regulates the oxidative status and antioxidant gene transcripts in CCl4-induced hepatotoxicity. PLoS ONE 2015, 10, e0121549. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Kim, Y.H.; Lee, Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Huang, Y.Y.; Duh, P.D.; Wu, S.C. Hepatoprotection of emodin and Polygonum multiflorum against CCl4-induced liver injury. Pharm. Biol. 2012, 50, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Yamamoto, K.; Kawabata, Y.; Yamada, S. In vitro/in vivo characterization of nanocrystalline formulation of tranilast with improved dissolution and hepatoprotective properties. Eur. J. Pharm. Biopharm. 2013, 85, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Jung, H.W.; Kim, Y.J.; Kwon, H.J.; Chae, H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern. Med. 2016, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, P. Hepatoprotective effect of curcumin on lindane-induced oxidative stress in male wistar rats. Toxicol. Int. 2011, 18, 124–129. [Google Scholar] [PubMed]
- Sankar, P.; Gopal Telang, A.; Kalaivanan, R.; Karunakaran, V.; Manikam, K.; Sarkar, S.N. Effects of nanoparticle-encapsulated curcumin on arsenic-induced liver toxicity in rats. Environ. Toxicol. 2015, 30, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem. Toxicol. 2008, 46, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Lee, H.Y.; Choi, M.K.; Chung, H.W.; Kim, S.W.; Chae, H.J. Protective effect of Curcuma longa L. Extract on CCl4-induced acute hepatic stress. BMC Res. Notes 2017, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lu, D.; Zou, Y.; Zhou, C.; Liu, H.; Tu, Cs.; Li, F.; Liu, L.; Zhang, S. Curcumin protects against intestinal origin endotoxemia in rat liver cirrhosis by targeting pcsk9. J. Food Sci. 2017, 82, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Kim, H.J.; Kim, J.K.; Chun, H.J.; Park, K. Preparation of redox-sensitive β-cd-based nanoparticles with controlled release of curcumin for improved therapeutic effect on liver cancer in vitro. J. Ind. Eng. Chem. 2017, 45, 156–163. [Google Scholar] [CrossRef]





| Parameters | Control | CCl4 | Cur 25 | Cur 50 | ηCur 25 | ηCur 50 |
|---|---|---|---|---|---|---|
| Convulsion | - | - | - | - | - | - |
| Salivation | - | - | - | - | - | - |
| Diarrhea | - | + | - | - | - | - |
| Mortality | - | - | - | - | - | - |
| Sedation | - | + | - | - | - | - |
| Skin irritation | - | - | - | - | - | - |
| CNS Depression | - | - | - | - | - | - |
| Body weight | - | + | + | + | - | - |
| Feed intake | - | + | - | - | - | - |
| Water intake | - | + | + | + | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marslin, G.; Prakash, J.; Qi, S.; Franklin, G. Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl4-Induced Subacute Hepatotoxicity in Wistar Rats. Polymers 2018, 10, 541. https://doi.org/10.3390/polym10050541
Marslin G, Prakash J, Qi S, Franklin G. Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl4-Induced Subacute Hepatotoxicity in Wistar Rats. Polymers. 2018; 10(5):541. https://doi.org/10.3390/polym10050541
Chicago/Turabian StyleMarslin, Gregory, Jose Prakash, Shanshan Qi, and Gregory Franklin. 2018. "Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl4-Induced Subacute Hepatotoxicity in Wistar Rats" Polymers 10, no. 5: 541. https://doi.org/10.3390/polym10050541
APA StyleMarslin, G., Prakash, J., Qi, S., & Franklin, G. (2018). Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl4-Induced Subacute Hepatotoxicity in Wistar Rats. Polymers, 10(5), 541. https://doi.org/10.3390/polym10050541